Abstract
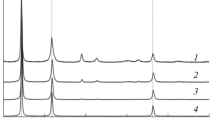
A new plasma-solution method of the CdO ultradisperse powders synthesis was described. The atmospheric pressure direct current discharge was excited in the ambient air by applying a high direct voltage to two pointed titanium electrodes placed above liquid anode and liquid cathode in the H-shaped cell. The discharge current was 40 mA and the total input power was about 40 W. The action of the DC glow discharge on the cadmium nitrate water solution in the absence of additional reagents and without electrodes-solution contact was shown to result in the production of the solids in the liquid phase. The kinetics of particles formation was studied using turbidimetry and nephelometry methods. Powders’ chemical composition and morphology was obtained using X-ray diffraction spectroscopy (XRD), electron-dispersive X-ray spectroscopy (EDX), thermogravimetric analysis (TGA), differential-scanning calorimetry (DSC) and scanning electron microscopy (SEM). It was found that as-synthesized powders are not the pure cadmium hydroxide but the mixture of the cadmium nitrate, hydroxy nitrate and hydroxide. Some assumptions regarding the mechanisms and pathway of the chemical processes both under the plasma action on the solution and during the calcination of as-synthesized powders were discussed.











Similar content being viewed by others
References
Li X, Gesset TA, Coutts T (2004) The properties of cadmium tin oxide thin-film compounds prepared by linear combinatorial synthesis. Appl Surf Sci 223(1–3):138–143
Hillie KT, Basson SS, Swart HC (2002) Effect of a CdO coating on the degradation of a ZnS thin film phosphor material. Appl Surf Sci 187(1–2):137–144
Subramanyam TK, Uthanna S, Naidu BS (1998) Preparation and characterization of CdO films deposited by dc magnetron reactive sputtering. Mater Lett 35(3–4):214–220
GulinoA Tabbi G (2005) CdO thin films: a study of their electronic structure by electron spin resonance spectroscopy. Appl Surf Sci 245(1–4):322–327
Maity R, Chattopadhyay KK (2006) Synthesis and characterization of aluminum-doped CdO thin films by sol–gel process. Sol Energy Mater Sol Cells 90(5):597–606
Seshadri A, de Tacconi NR, Chenthamarakshan CR, Rajeshwar K (2006) Cathodic electrodeposition of CdO thin films from oxygenated aqueous solutions. Electrochem Solid State Lett 9(1):C1–C4
Lokhande BJ, Patil PS, Uplane MD (2004) Studies on cadmium oxide sprayed thin films deposited through non-aqueous medium. Mater Chem Phys 84(2–3):238–242
Subramanyam TK, Srinivasulu NB, Uthanna S (2001) Studies on dc magnetron sputtered cadmium oxide films. Appl Surf Sci 169–170:529–534
Bhosale CH, Kambale AV, Kokate AV, Rajpure KY (2005) Structural, optical and electrical properties of chemically sprayed CdO thin films. Mater Sci Eng, B 122(1):67–71
Ristic M, Popovic S, Music S (2004) Formation and properties of Cd(OH)2 and CdO particles. Mater Lett 58(20):2494–2499
Ghosh PK, Das S, Chattopadhyay KK (2005) Temperature dependent structural and optical properties of nanocrystalline CdO thin films deposited by sol–gel process. J Nanoparticle Res 7(2–3):219–225
Zhou Q, Ji Z, Hu B, Chen C, Zhao L, Wang C (2007) Low resistivity transparent conducting CdO thin films deposited by DC reactive magnetron sputtering at room temperature. Mater Lett 61(2):531–534
Gujara TP, Shinde VR, Kim W-Y, Jung K-D, Lokhande CD, Joo O-S (2008) Formation of CdO films from chemically deposited Cd(OH)2 films as a precursor. Appl Surf Sci 254(13):3813–3818
Zhao Z, Morel DL, Ferekides CS (2002) Electrical and optical properties of tin-doped CdO films deposited by atmospheric metalorganic chemical vapor deposition. Thin Solid Films 413(1–2):203–211
Gulino A, Castelli F, Dapporto P, Rossi P, Fragala I (2002) Synthesis and characterization of thin films of cadmium oxide. Chem Mater 14(2):704–709
Gurumurugan K, Mangalaraj D, Narayandass SK, Sekar K, Girija Vallabhan CP (1994) Characterization of transparent conducting CdO films deposited by spray pyrolysis. Semicond Sci Technol 9(10):1827–1832
Han X, Liu R, Xu Z, Chen W, Zheng Y (2005) Room temperature deposition of nanocrystalline cadmium peroxide thin film by electrochemical route. Electrochem Commun 7(12):1195–1198
Askarinejad A, Morsali A (2009) Synthesis of cadmium(II) hydroxide, cadmium(II) carbonate and cadmium(II) oxide nanoparticles; investigation of intermediate products. Chem Ing J 150(1):569–571
Naje AN, Abbas LK, Salman G, Abdullah ET (2014) Current–voltage characteristics of CdO nanostructure ultraviolet photoconductive detector. Int J Sci Env Tech 3(2):684–691
Chen Q, Li J, Li Y (2015) A review of plasma–liquid interactions for nanomaterial synthesis. J Phys D Appl Phys 48(42):424005
Saito G, Akiyama T (2015) Nanomaterial synthesis using plasma generation in liquid. J Nanomater. Article ID 123696
Shutov DA, Rybkin VV, Ivanov AN, Smirnova KV (2017) Synthesis of zinc oxide powders in plasma-solution systems. High Energy Chem 51(1):65–69
Altomare A, Corriero N, Cuocci C, Falcicchio A, Moliterni A, Rizzi R (2015) QUALX2.0: a qualitative phase analysis software using the freely available database POW_COD. J Appl Cryst 48(2):598–603
Grazulis S, Daskevic A, Merkys A, Chateigner D, Lutterotti L, Quiros M, Serebryanaya NR, Moeck P, Downs RT, LeBail A (2012) Crystallography open database (COD): an open-access collection of crystal structures and platform for world-wide collaboration. Nucl Acids Res 40:D420–D427
Lawler DM (2005) Spectrophotometry: turbidimetry and nephelometry. Book: encyclopedia of analytical science, vol 2. Elsevier, Amsterdam, pp 343–351
Shutov DA, Rybkin VV, Smirnov SA (2014) The expediency of taking account of the flux of neutral plasma species in simulation of liquid-phase processes initiated by DC glow discharge. High Energy Chem 48(6):391
Hart EJ, Anbar M (1962) Absorption spectrum of the hydrated electron in water and in aqueous solutions. J Am Chem Soc 84(21):4090–4095
Buxton GV, Greenstock CL, Helman WP, Ross ABJ (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. Phys Chem Ref Data 17:513–886
Kelm M, Lilie J, Hengle A (1975) Pulse radiolytic investigation of the reduction of cadmium(II) ions. J Chem Soc Faraday Trans 1(71):1132–1142
Padeste C, Schmalle HW, Oswald HR (1992) Crystal structure of calcium hydroxide nitrate hydrate and its superstructure in relation to cadmium hydroxide nitrate hydrate. Z Kristallographie 200:35–46
Riou A, Gerault Y, Cudennec Y (1990) Etude structurale de γ-Cd(OH)2 ou Cd2O(OH)2(H2O). Mater Res Bull 25:987–996
Soleimani F, Aghaiel H, Gharib F (2008) Hydrolysis of cadmium cation in different ionic strength. J Phys Theor Chem 5(2):73–78
Shi W, Wang C, Wang H, Zhang H (2006) Hexagonal nanodisks of cadmium hydroxide and oxide with nanoporous structure. Cryst Growth Des 6(4):915–918
Ivanov VV, Shubin AA, Irtugo LA (2009) The CdO powders synthesis by a thermal decomposition of unstable salts for arcing electro contact materials. J Sib Fed Univ Eng Technol 4:409–417
Wojciechowski KT, Maøecki A (1999) Mechanism of thermal decomposition of cadmium nitrate Cd(NO3)2·4H2O. Thermochim Acta 331(1):73–77
Rodriguez Roldan M, Auffrédic J-P, Louër D (1983) Influence de la structure cristalline sur la décomposition thermique des hydroxynitrates de metaux bivalents: exemple des hydroxy-nitrates de cadmium. J Therm Anal 26(1):131–138
Srivastava OK, Secc EA (1967) Studies on metal hydroxy compounds. III. Thermal analyses of cadmium derivatives Cd(OH)2, CdOHCl, and CdOHF. Can J Chem 45(12):1375–1378
Tauc J (1968) Optical properties and electronic structure of amorphous Ge and Si. Mater Res Bull 3(1):37–46
Davis EA, Mott NF (1970) Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos Mag A 22(179):903–922
Brouwer AM (2011) Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl Chem 83(12):2213–2228
Benko FA, Koffyberg FP (1986) Quantum efficiency and optical transitions of CdO photoanodes. Solid States Commun 57(12):901–903
Acknowledgements
This study was supported by Ministry of Education and Science of the Russian Federation, Project 3.1371.2017/.4.6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shutov, D.A., Smirnova, K.V., Gromov, M.V. et al. Synthesis of CdO Ultradisperse Powders Using Atmospheric Pressure Glow Discharge in Contact With Solution and the Investigation of Intermediate Products. Plasma Chem Plasma Process 38, 107–121 (2018). https://doi.org/10.1007/s11090-017-9856-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-017-9856-0