Abstract
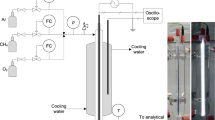
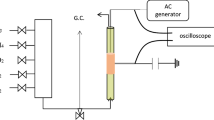
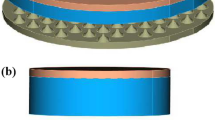
A continuous plug flow reactor supported by a dielectric barrier discharge (DBD) is used to study the conversion of methane, carbon dioxide, and oxygen at different compositions. The three studied gases were diluted with helium to 3 % with an overall flow rate of 200 sccm. The 13.56 MHz plasma was ignited at atmospheric pressure. The product stream and the inlet flow were analyzed by a FTIR spectrometer equipped with a White-cell and by a quadrupole mass spectrometer. The DBD reactor generates hydrogen, carbon monoxide, ethane, ethene, acetylene, formaldehyde, and methanol. Additional oxygen in the feed has positive effects on the yield of methanol, formaldehyde and carbon monoxide and reduces the total consumed energy. The hydrogen yield reaches its maximum at medium amounts of oxygen in the inlet flow. The conversion of methane increases to a limiting value of about 35 %. Methane rich feeds increase the yield of hydrogen, ethane and methanol. On the other hand, additional oxygen has a negative influence on the produced amount of C2 hydrocarbons. The conversion of methane and carbon dioxide as well as the yield of synthesis gas components and C2 hydrocarbons increases by changing the plasma power to higher values.












Similar content being viewed by others
References
Harding RH, Peters AW, Nee JRD (2001) Appl Catal. A 221:389–396
Rasi S, Veijanen A, Rintala J (2007) Energy 32:1375–1380
Rasi S, Läntelä J, Veijanen A, Rintala J (2008) Waste Manage 28:1528–1534
Tao X, Bai M, Li X, Long H, Shang S, Yin Y, Dai X (2011) Prog Energy Combust 37:113–124
Kogelschatz U, Eliasson B, Egli WJ (1997) Phys IV France 07:47–66
Kroker T, Kolb T, Schenk A, Krawczyk K, Młotek M, Gericke K-H (2012) Plasma Chem Plasma Process 32:565–582
Sentek J, Krawczyk K, Młotek M, Kalczewska M, Kroker T, Kolb T, Schenk A, Gericke K-H, Schmidt-Szałowski K (2010) Appl Catal B 94:19–26
Zhang K, Eliasson B, Kogelschatz U (2002) Ind Eng Chem Res 41:1462–1468
Song HK, Lee H, Choi J-W, Na B (2004) Plasma Chem Plasma Process 24:57–72
Wang Q, Yan BH, Jin Y, Cheng Y (2009) Energy Fuels 23:4196–4201
Li X-S, Zhua A-M, Wang K-J, Xu Y, Song Z-M (2004) Catal Today 98:617–624
Kolb T, Kroker T, Gericke K-H (2013) Vaccum 88:144–148
Kroker T, Kolb T, Krawczyk K, Mlotek M, Schenk A, Gericke K-H (2010) Front Appl Plasma Technol 3:69–73
Li Y, Liu C-J, Eliasson B, Wang Y (2002) Energy Fuels 16:864–870
Pietruszka B, Heintze M (2004) Catal Today 90:151–158
Larkin DW, Lobban LL, Mallinson RG (2001) Catal Today 71:199–210
Aghamir FM, Matin NS, Jalili AH, Esfarayeni MH, Khodagholi MA, Ahmadi R (2004) Plasma Sources Sci Technol 13:707–711
Zhou LM, Xue B, Kogelschatz U, Eliasson B (1998) Plasma Chem Plasma Process 18:375–393
Nozaki T, Goujard V, Yuzawa S, Moriyama S, Agiral A, Okazaki K (2011) J Phys D Appl Phys 44:1–6
Larkin DW, Caldwell TA, Lobban LL, Mallinson RG (1998) Energy Fuels 12:740–744
Baowei W, Xu Z, Yongwei L, Genhui X (2008) J Nat Gas Chem 18:94–97
Darwent BD (1970) Bond dissociation energies in simple molecules. NBSDS-NBS 31
Beller M, Cornils B, Frohning CD, Kohlpaintner CW (1995) J Mol Catal A Chem 104:17–85
Iglesia E (1997) Appl Catal A 161:59–78
Liu C-J, Mallinson R, Lobban LJ (2009) Catalysis 179:326–334
Tsang W, Hampson RF (1986) J Phys Chem Ref Data 15:1087–1279
Kolb T, Kroker T, Voigt JH, Gericke K-H (2012) Plasma Chem Plasma Process. doi:10.1007/s11090-012-9411-y
Acknowledgments
This project is part of the framework of the European Research Area (ERA) Chemistry call. The work is financially supported by the Deutsche Forschungsgemeinschaft (DFG). Support by the IGSM Braunschweig is gratefully acknowledged. We acknowledge T. Kroker for measuring the impedance spectra of our reactor and C. Maul for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolb, T., Voigt, J.H. & Gericke, KH. Conversion of Methane and Carbon Dioxide in a DBD Reactor: Influence of Oxygen. Plasma Chem Plasma Process 33, 631–646 (2013). https://doi.org/10.1007/s11090-013-9448-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-013-9448-6