Abstract
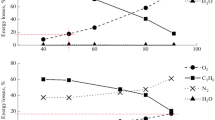
Oxidation of propylene with oxygen, air and a mixture of nitrogen–oxygen in a barrier discharge is investigated. The selectivity towards formation of propylene oxide in pure oxygen is shown to be as high as 45 wt% and the propylene conversion ratio is found to be 12.9 wt%. In the oxidation with air, the propylene oxide selectivity is 23 wt%, while the conversion is 7.5 wt%. The values of propylene conversion and selectivity towards formation of propylene oxide in a barrier discharge are consistent with those obtained by the thermocatalytic methods for production of propylene oxide.








Similar content being viewed by others
References
Nijhuis AT, Makkee M, Moulijn JA, Weckhuysen BM (2006) Ind Eng Chem Res 45:3447–3459
Pat. 5698719 US, US Cl. 549/534. Oxirane production
Jin G, Lu G, Guo Y, Guo Yu, Wangle J, Lui X (2003) Catal Lett 87:249–252
Suoa Z, Jina M, Lub J, Weib Z, Lib C (2008) J Nat Gas Chem 17:184–190
Luo M, Lu J, Li C (2003) Catal Lett 86:43–49
Lu J, Luo M, Lei H, Li C (2002) Appl Catal A 237:11–19
Sreethawong T, Suwannabart T, Chavadej S (2008) Plasma Chem Plasma Process 26:629–642
Kudryashov SV, Shchegoleva GS, Sirotkina EE, Ryabov AY (2000) High Energy Chem 34:112–115
Hyduk W, Wong C-F (1990) Can J Chem Eng 68:653–660
Suhr H (1983) Plasma Chem Plasma Process 31:1–61
Suhr H, Schmid H, Pfeundschuh H, Iacossa D (1984) Plasma Chem Plasma Process 4:285–295
http://kinetics.nist.gov/kinetics/Detail?id=2004DUN/RAV2152-2161:9. Accessed on 9 June 2011
http://kinetics.nist.gov/kinetics/Detail?id=1997DEM/SAN1-266:428. Accessed on 9 June 2011
http://kinetics.nist.gov/kinetics/Detail?id=1987CVE261:32. Accessed on 9 June 2011
http://kinetics.nist.gov/kinetics/Detail?id=1979KAJ/FUE445:3. Accessed on 9 June 2011
Hirokami S, Cvetanovic R (1974) J Am Chem Soc 96:3738–3746
Tanner D, Kandanarachchi P (1998) J Org Chem 63:4587–4593
Kajimoto O, Yamasaki H, Fueno T (1979) Chem Phys Lett 68:127–130
Fresnet F, Pasquiers S, Postel C, Puech V (2002) J Phys D Appl Phys 35:882–890
Makulski W, Gawlowski J, Niedzielski J (1981) J Phys Chem 85:2950–2955
http://kinetics.nist.gov/kinetics/Detail?id=1993JEN/MUR433-446:5. Accessed on 9 June 2011
http://kinetics.nist.gov/kinetics/Detail?id=1996BOY/NOZ201-206:2. Accessed on 9 June 2011
http://kinetics.nist.gov/kinetics/Detail?id=1991TSA221-273:32. Accessed on 9 June 2011
Fridman A (2008) Plasma chemistry. Cambridge university press, New York
http://www.bolsig.laplace.univ-tlse.fr/. Accessed on 18 May 2011
Moreau N, Pasquiers S, Blin-Simiand N, Magne L, Jorand F, Postel C, Vacher J-R (2010) J Phys D Appl Phys 43:285201
DeBoer GD, Dodd JA (2007) J Phys Chem A 111:12977–12984
Acknowledgments
The authors would like to thank Alexey I. Suslov from the Institute of High Current Electronics, SB RAS for assistance in interpretation of electric parameters of barrier discharge.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kudryashov, S., Ochered’ko, A., Ryabov, A. et al. Oxidation of Propylene with Oxygen and Air in a Barrier Discharge in the Presence of Octane. Plasma Chem Plasma Process 31, 649–661 (2011). https://doi.org/10.1007/s11090-011-9318-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-011-9318-z