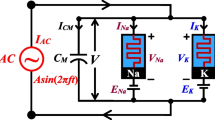
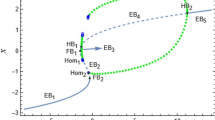
Abstract
The bursting discharge behaviour of neurons is affected by many factors, among which temperature is one of the more important factors. In this work, we study the bursting discharge behaviour and dynamics process of two different temperature-sensitive ion channels, the temperature-sensitive potassium current and the temperature-sensitive calcium current. In the case of the temperature-sensitive potassium current, the bursting discharge waveforms, codimension-1 bifurcations and trajectory plots at different temperatures indicate that five different types of bursting discharge (Hopf/Flip, Hopf/Homoclinic, Fold/Homoclinic, Fold/Fold Cycle, Circle/Big Homoclinic) appear with increasing temperature. In the case of temperature-sensitive calcium current, two types of bursting discharge (Circle/Big Homoclinic, Fold/Fold Cycle) emerge. According to the bursting discharge waveforms, the rise in temperature can promote the generation of bursting discharge at the beginning, and finally, the bursting discharge phenomenon disappears. This is consistent with the experimental results that blocking potassium and calcium currents can promote the bursting of Purkinje neurons. Then, it can be seen from the codimension-2 bifurcation and the waveform area distribution diagrams that even if the dynamic paths are consistent, the bursting discharge types and the waveforms may be different. In contrast, even if the bursting discharge type is the same, the dynamic paths and the waveform may be different. These results provide insight into the effect of temperature on the neuronal dynamics and bursting behaviour of temperature-sensitive ion channels.











Similar content being viewed by others
Data availability
Data available on request from the authors.
References
Cooper, D.C.: The significance of action potential bursting in the brain reward circuit. Neurochem. Int. 41(5), 333–340 (2002)
Jefferys, J.G.R., Haas, H.L.: Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature 300(5891), 448–450 (1982)
Li, Y.Y., Gu, H.G., Jia, Y.B., et al.: Fast-slow variable dissection with two slow variables related to calcium concentrations: a case study to bursting in a neural pacemaker model. Nonlinear Dyn. 107(1), 1223–1245 (2022)
Lu, B., Gu, H.G., Wang, X.J., et al.: Paradoxical enhancement of neuronal bursting response to negative feedback of autapse and the nonlinear mechanism. Chaos Soliton Fract. 145, 110817 (2021)
Guan, L.N., Gu, H.G., Zhao, Z.G.: Paradoxical enhancement of neuronal bursting response to negative feedback of autapse and the nonlinear mechanism. Nonlinear Dyn. 104(1), 577–601 (2021)
Smith, J.C., Ellenberger, H.H., Ballanyi, K., et al.: Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254(5032), 726–729 (1991)
Onimaru, H., Arata, A., Homma, I.: Intrinsic burst generation of preinspiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Exp. Brain Res. 106(1), 57–68 (1995)
Lin, H.R., Wang, C.H., Chen, C.J., et al.: Neural bursting and synchronization emulated by neural networks and circuits. IEEE Trans. Circuits I. 68(8), 3397–3410 (2021)
Postnova, S., Voigt, K., Braun, H.A.: Neural synchronization at tonic-to-bursting transitions. J. Biol. Phys. 33(2), 129–143 (2007)
Yao, Z., Ma, J., Yao, Y.G., et al.: Synchronization realization between two nonlinear circuits via an induction coil coupling. Nonlinear Dyn. 96(1), 205–217 (2019)
Ma, J., Wu, F.Q., Alsaedi, A., et al.: Crack synchronization of chaotic circuits under field coupling. Nonlinear Dyn. 93(4), 2057–2069 (2018)
Wu, T.W., Zhang, X.H., Liu, Z.H.: Understanding the mechanisms of brain functions from the angle of synchronization and complex network. Front. Phys. 17(3), 1–23 (2022)
Cao, H.Y., Liu, Z.H.: A novel synchronization transition and amplitude death in the local brain networks of cortical regions. Nonlinear Dyn. 1–14 (2022)
Kepecs, A., Wang, X.J., Lisman, J.: Bursting neurons signal input slope. J. Neurosci. 22(20), 9053–9062 (2002)
Kepecs, A., Lisman, J.: Information encoding and computation with spikes and bursts. Network-Comput. Neural. 14(1), 103 (2003)
Guo, Y.T., Zhou, P., Yao, Z., et al.: Biophysical mechanism of signal encoding in an auditory neuron. Nonlinear Dyn. 105(4), 3603–3614 (2021)
Prince, D.A.: Neurophysiology of epilepsy. Annu. Rev. Neurosci. 1(1), 395–415 (1978)
Traub, R.D., Wong, R.K.S.: Cellular mechanism of neuronal synchronization in epilepsy. Science 216(4547), 745–747 (1982)
Marder, E.: Motor pattern generation. Curr. Opin. Neurobiol. 10(6), 691–698 (2000)
Butera, J., Robert, J., Rinzel, J., et al.: Models of respiratory rhythm generation in the pre-Botzinger complex. I. Bursting pacemaker neurons. J. Neurophysiol. 82(1), 382–397 (1999)
Butera, J., Robert, J., Rinzel, J., et al.: Models of respiratory rhythm generation in the pre-Botzinger complex. II. Populations of coupled pacemaker neurons. J. Neurophysiol. 82(1), 398–415 (1999)
Izhikevich, E.M.: Dynamical systems in neuroscience. MIT Press, Cambridge (2007)
Ma, K.H., Gu, H.G., Zhao, Z.G.: Fast-slow variable dissection with two slow variables: a case study on bifurcations underlying bursting for seizure and spreading depression. Int. J. Bifurcat. Chaos 31(06), 2150096 (2021)
Hua, H.T., Gu, H.G., Jia, Y.B., et al.: The nonlinear mechanisms underlying the various stochastic dynamics evoked from different bursting patterns in a neuronal model. Commun. Nonlinear Sci. 110, 106370 (2022)
Wang, X.J., Gu, H.G., Lu, B.: Paradoxical reduction and the bifurcations of neuronal bursting activity modulated by positive self-feedback. Nonlinear Dyn. 101(4), 2383–2399 (2020)
Rinzel, J.: Ordinary and Partial Differential Equations. Springer, Berlin (1985)
Rinzel, J.: Mathematical Topics in Population Biology, Morphogenesis and Neurosciences. Springer, Berlin (1987)
Izhikevich, E.M.: Neural excitability, spiking and bursting. Int. J. Bifurcat. Chaos 10(06), 1171–1266 (2000)
Huang, Y., Ko, H., Cheung, Z.H., et al.: Dual actions of brain-derived neurotrophic factor on GABAergic transmission in cerebellar Purkinje neurons. Exp. Neurol. 233(2), 791–798 (2012)
Stay, T.L., Laurens, J., Sillitoe, R.V., Angelaki, D.E.: Genetically eliminating Purkinje neuron GABAergic neurotransmission increases their response gain to vestibular motion. Proc. Natl. Acad. Sci. 116(8), 3245–3250 (2019)
Rulkov, N.F.: Modeling of spiking-bursting neural behavior using two-dimensional map. Phys. Rev. E 65(4), 041922 (2002)
Bashkirtseva, I., Nasyrova, V., Ryashko, L.: Stochastic spiking-bursting excitability and transition to chaos in a discrete-time neuron model. Int. J. Bifurcat. Chaos 30(10), 2050153 (2020)
Yazdi, H.H., Janahmadi, M., Behzadi, G.: The role of small-conductance Ca\(^{2+}\)-activated K\(^{+}\) channels in the modulation of 4-aminopyridine-induced burst firing in rat cerebellar Purkinje cells. Brain Res. 1156, 59–66 (2007)
Womack, M.D., Hoang, C., Khodakhah, K.: Large conductance calcium-activated potassium channels affect both spontaneous firing and intracellular calcium concentration in cerebellar Purkinje neurons. Neuroscience 162(4), 989–1000 (2009)
Womack, M., Khodakhah, K.: Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J. Neurosci. 22(24), 10603–10612 (2002)
Xu, Y., Guo, Y.Y., Ren, G.D., et al.: Dynamics and stochastic resonance in a thermosensitive neuron. Chaos 385, 125427 (2020)
Zhang, X.C., Liu, S.Q., Ren, H.X., et al.: Dynamic properties of Purkinje cells having different electrophysiological parameters: a model study. Neurophysiology 47(1), 2–10 (2015)
Ermentrout, B.: Simulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students. SIAM, Philadelphia (2002)
De Schutter, E., Bower, J.M.: An active membrane model of the cerebellar Purkinje cell II. Simulation of synaptic responses. J. Neurophysiol. 71, 401–419 (1994)
Khaliq, Z.M., Gouwens, N.W., Raman, I.M.: The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J. Neurosci. 23, 4899–4912 (2003)
Kramer, M.A., Traub, R.D., Kopell, N.J.: New dynamics in cerebellar purkinje cells: torus canards. Phys. Rev. Lett. 101(6), 068103 (2008)
Benes, G.N., Barry, A.M., Kaper, T.J., et al.: An elementary model of torus canards. Chaos 21(2), 023131 (2011)
Grandl, J., Kim, S.E., Uzzell, V., et al.: Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat. Neurosci. 13(6), 708–714 (2010)
Rinberg, A., Taylor, A.L., Marder, E.: The effects of temperature on the stability of a neuronal oscillator. PLoS Comput. Biol. 9(1), e1002857 (2013)
Womack, M.D., Khodakhah, K.: Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons. J. Neurosci. 23(7), 2600–2607 (2003)
Womack, M.D., Chevez, C., Khodakhah, K.: Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J. Neurosci. 24(40), 8818–8822 (2004)
Isope, P., Hildebrand, M.E., Snutch, T.P.: Contributions of T-type voltage-gated calcium channels to postsynaptic calcium signaling within Purkinje neurons. Cerebellum 11(3), 651–665 (2012)
Etémé, A.S., Tabi, C.B., Mohamadou, A.: Firing and synchronization modes in neural network under magnetic stimulation. Commun. Nonlinear Sci. 72, 432–440 (2019)
Etémé, A.S., Tabi, C.B., Beyala Ateba, J.F., et al.: Chaos break and synchrony enrichment within Hindmarsh-Rose-type memristive neural models. Nonlinear Dyn. 105(1), 785–795 (2021)
Etémé, A.S., Tabi, C.B., Ateba, J.F.B., et al.: Neuronal firing and DNA dynamics in a neural network. J. Phys. Commun. 2(12), 125004 (2018)
Xu, Y., Ma, J.: Control of firing activities in thermosensitive neuron by activating excitatory autapse. Chin. Phys. B 30(10), 100501 (2021)
Qin, H.X., Ma, J., Jin, W.Y., et al.: Dynamics of electric activities in neuron and neurons of network induced by autapses. Sci. China Technol. Sc. 57(5), 936–946 (2014)
Mortensen, L.S., Schmidt, H., Farsi, Z., et al.: K\(_{V}\)10.1 opposes activity-dependent increase in Ca\(^{2+}\) influx into the presynaptic terminal of the parallel fibre-Purkinje cell synapse. Cerebellum 593(1), 181–196 (2015)
Xing, M.M., Song, X.L., Yang, Z.Q., et al.: Bifurcations and excitability in the temperature-sensitive Morris–Lecar neuron. Nonlinear Dyn. 100(3), 2687–2698 (2020)
Funding
This work was supported by the National Natural Science Foundation of China with Grant No. 11872084 (ZQY), No. 12075017 (YC)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there are no any conflict with the publication of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xing, M., Yang, Z. & Chen, Y. Bursting types and bifurcation analysis of the temperature-sensitive Purkinje neuron. Nonlinear Dyn 111, 1819–1834 (2023). https://doi.org/10.1007/s11071-022-07917-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-022-07917-2