Abstract
Olfactory training (OT), or smell training,consists of repeated exposure to odorants over time with the intended neuroplastic effect of improving or remediating olfactory functioning. Declines in olfaction parallel declines in cognition in various pathological conditions and aging. Research suggests a dynamic neural connection exists between olfaction and cognition. Thus, if OT can improve olfaction, could OT also improve cognition and support brain function? To answer this question, we conducted a systematic review of the literature to determine whether there is evidence that OT translates to improved cognition or altered brain morphology and connectivity that supports cognition. Across three databases (MEDLINE, Scopus, & Embase), 18 articles were identified in this systematic review. Overall, the reviewed studies provided emerging evidence that OT is associated with improved global cognition, and in particular, verbal fluency and verbal learning/memory. OT is also associated with increases in the volume/size of olfactory-related brain regions, including the olfactory bulb and hippocampus, and altered functional connectivity. Interestingly, these positive effects were not limited to patients with smell loss (i.e., hyposmia & anosmia) but normosmic (i.e., normal ability to smell) participants benefitted as well. Implications for practice and research are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Loss of smell can occur for numerous reasons including nasal or sinus infection, chemical exposure and pollutants, dental problems, medication use, chronic conditions such as diabetes and hypertension, traumatic brain injury, neurodegenerative diseases such as Parkinson’s disease, or normal aging (Doty, 2019). A partial loss of smell (hyposmia) or a complete loss of smell (anosmia) detrimentally impacts quality of life (i.e., loss of favored smells associated with food, activities, and sex), poses a safety risk (i.e., eating spoiled foods, unable to detect harmful fumes), and can be debilitating for people, especially those who rely on this sensory ability in their professional lives (i.e., natural gas workers, chefs, perfumers). In the National Social Life, Health, and Aging Project, a 15-year longitudinal study of older adults, Eliyan et al. (2020) found that baseline olfactory impairments predicted depression 5–10 years later. In the same study, Pinto et al. (2014) discovered that olfactory impairment significantly predicted 5-year mortality (OR = 3.37).
Interestingly, declining olfaction and olfactory impairment serve as a bellwether for the development of cognitive impairment and neurodegenerative diseases. In a meta-analysis of 12 articles examining olfactory function (e.g., odor identification, odor discrimination, or odor detection threshold) including 788 patients with mild cognitive impairment (MCI) and 563 patients with Alzheimer’s disease (AD), Jung et al. (2019) found that olfactory impairment, specifically odor identification, was more profound in those with AD. This finding suggests that a simple odor identification test may discriminate between MCI and AD. Similarly, in a meta-analysis of 31 articles examining olfactory function in 1,993 MCI patients and 2,861 cognitively healthy older adults, Roalf et al. (2017) found that olfactory impairment, specifically odor identification, was more severe in those with MCI. This bellwether effect is observed in other health conditions as well. In fact, many studies reported the association between olfactory impairment and corresponding cognitive impairments in normal middle-aged and older adults (Adams et al., 2018; Devanand, 2016; Woodward et al., 2017, 2018). Studies have also reported that poor olfaction in cognitively normal older adults is associated with future risk of dementia at longitudinal follow-up (Devanand et al., 2015; Schubert et al., 2008; Yaffe et al., 2017). These studies suggest a potentially strong neurological connection between olfaction and cognition.
Unlike the other sensory systems that are gated through the thalamus, the peripheral olfactory system extends neuronal projections directly to cortical areas that support cognition such as the orbitofrontal cortex, amygdala, pyriform cortex, and entorhinal cortex (Leon & Woo, 2018; See Fig. 1). Remarkably, compromised olfaction or a loss of olfaction corresponds to volume loss in the same brain regions (Leon & Woo, 2018; Yao et al., 2014). Olfaction and localized loss of brain volume show a parallel vulnerability to deterioration with age (Kollndorfer et al., 2015; Segura et al., 2013).
An extensive literature clearly demonstrates that intentional odorant delivery can negatively or positively impact cognitive function (Johnson, 2011). In a classic study, Rotton (1983) found that exposure to a malodor (ethanethiol) negatively affected proofreading (a complex task) but not basic arithmetic (a simple task). Others have found that exposure to commercially available essential oils in real-time can improve memory, alertness, vigilance, self-perception, pain perception, and mood (Johnson, 2011). Similarly, Tsushima et al. (2021) found that exposure to a lemon odorant and vanilla odorant modulated positively and negatively, respectively, low-level visual perception, which suggests some innate characteristics of odorants on perception and cognitive function.
Following this reasoning, neuroplastic processes may be upregulated through olfactory stimulation; such olfactory stimulation provides novelty and adaptation, thus potentially supporting cognitive function. In several studies, more stimulating environments, across factors such as occupational complexity, diverse work histories (Carr et al., 2020), exposure and mastery of another language (Bialystok et al., 2004; Kuhl et al., 2016), or engaging in challenging activities such as computerized cognitive training (Lampit et al., 2014), can improve cognition and change brain morphology. It is well accepted that enriched environments may enhance cognitive reserve, which protects one from cognitive decline and dementia (Vance et al., 2019). It has been hypothesized that sensory stimulation, such as olfactory training (OT; a.k.a., smell training), could upregulate neuroplastic processes to improve cognition, brain connectivity, and brain health (Leon & Woo, 2018).
When we consider the positive role of sensory stimulation (i.e., visual, auditory, tactile, gustatory, and olfactory) or the negative role of sensory deprivation or impairment of sensory abilities, the potential impact on neuroplasticity becomes apparent (Leon & Woo, 2018). Moreover, in a sample of 3,005 community-dwelling older adults, Pinto et al. (2017) found that global sensory impairment in all five senses at baseline independently predicted decreased physical and cognitive function and increased significant weight loss and mortality five years later. Based on the Common Cause Hypothesis, the rate at which our sensory abilities decline parallels that of cognitive declines (Dulay & Murphy, 2002; Uchida et al., 2019). In the Victoria Longitudinal Study of Aging (N = 408), MacDonald et al. (2018) observed that olfaction, surprisingly more than vision and hearing, was predictive of cognitive decline; others have found similar associations between olfaction and cognition (Dulay & Murphy, 2002). Importantly, studies have demonstrated cognition improved once hearing (Brewster et al., 2021; Uchida et al., 2019), vision (Pellegrini et al., 2020; Varadaraj et al., 2021), and olfaction (Birte-Antina et al., 2018) are improved via mechanical devices (i.e., hearing aids; Knopke & Olze, 2018), surgery (i.e., cataract surgery; Kheirkhan et al., 2018), or OT (Birte-Antina et al., 2018; Knudsen et al., 2015), respectively. Yet, the extent of this association has not been well assessed in the case of OT.
Given the connection between olfaction and cognition, does OT improve cognition and alter brain structure and connectivity? In other words, does peripheral stimulation of the olfactory system (smelling odorants through OT) produce changes in cognition and brain morphology or function. In this systematic review, we addressed this question by surveying the extant literature systematically and documenting whether OT improves cognition, or brain resources (i.e., morphology, function, connectivity) that support cognition. Second, the identified articles (N = 18) were briefly summarized (Appendix 1). Third, a synthesis of these articles was conducted. Finally, implications for practice and future research directions were discussed.
Methodology of Systematic Review
Using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) approach (Moher et al., 2009), on January 19, 2022, MEDLINE (via PubMed), Embase, and Scopus databases were searched for research studies on any type of OT tested in humans, of which cognitive or brain neuroimaging outcome data were gathered and reported (Fig. 2); a year restriction was not imposed. Search terms are provided in Table 1. From this, 1,015 records were identified and 82 duplicates were removed, leaving 933 records to be reviewed. An additional search was conducted in September 7, 2022 to include newer publications not captured from the original January search. The search update resulted in 66 new records, of which 0 were deemed relevant to be included in the final review. Using the Covidence software, two of the authors (DEV & JSF) reviewed these article titles, abstracts, and articles separately to determine whether the article met study inclusion criteria. They then compared their findings and discussed each one until consensus was met on whether the article met the criteria.
More precisely, the articles (in English) were evaluated for the following inclusion criteria: 1) original research studies in adult humans (not systematic reviews, review articles, or case reports); 2) examination of any standard OT (repeated odorant exposure) occurring over multiple sessions; 3) experimental study design with a clear baseline and posttest follow-up assessment; 4) olfaction measured at least at baseline; and 5) neuronal (e.g., MRI, EEG brain neuroimaging, cerebral blood flow, neurotropic factors) or cognitive (e.g., cognitive testing) outcomes must had been assessed at least at baseline and at one follow-up assessment. Studies that did not meet all five inclusion criteria were excluded.
OT Intervention Studies
As a level 1a evidence supported therapy, OT is considered as an effective approach to restore or improve olfaction (Patel, 2017). Despite the large number of OT studies, OT studies that include cognitive or neural (e.g., brain neuroimaging) outcomes have only recently becoming more common. For the purposes of this review, it is important to highlight the basic structure of most OT protocols found in the literature; however, there is a great deal of variability in how OT is delivered. OT studies have four essential elements: 1) participant selection, 2) targeted outcomes, 3) intervention components, and 4) treatment adherence. First, many studies targeted participants with objective or subjective olfactory impairment or those vulnerable for developing such impairment (e.g., older adults); however, some studies attempted to improve olfaction in normosmic participants. Second, subjective olfaction is measured by self-reported ability to smell (e.g., smell complaints, ability to smell) while several aspects of objective olfaction are often reported including: a) odor detection threshold (i.e., detecting an odorant among three odorant pens with the other two pens containing an odorless solvent), b) odor discrimination (i.e., being able to discriminate the unique odorant from otherwise a choice of identical odorants), and c) odor identification tests (i.e., smelling an odorant and identifying it from a list of four descriptors). In fact, many studies combine these three measures to form a composite score referred to as TDI (i.e., odor detection Threshold, odor Discrimination, and odor Identification). Third, OT entails exposure to various odorants on a regular basis and consists of the following parameters: a) delivery vehicle, b) odorants, and c) dosage. The delivery vehicle varies from study to study but typically has odorants of various concentrations placed in small capsules or bottles in which the cap is removed for the participant to sniff. In earlier human studies with OT, the odorants and their concentrations were of approximately equal and moderately perceived intensity (Livermore & Laing, 1996). Odorants typically consist of four primary types considered to be representative across the “odor prism” including: a) flowery (e.g., rose), b) resinous (e.g., eucalyptus), c) aromatic (e.g., cloves), and d) fruity (e.g., lemon); however, some studies may actually have as many as 12 odorants (e.g., Altundag et al., 2015) or vary the odorants between single-molecule to complex-molecule odorants or “light weight molecules” versus “heavy weight molecules” (e.g., Poletti et al., 2017). Dosage varies greatly but generally consists of smelling each odorant for 10–20 s at a time, 2x/day over a period of 8–35 weeks. Finally, concerning adherence, treatment adherence is commonly assessed via a daily diary with results collected at the end of training (e.g., Birte-Antina et al., 2018; Knolldorfer et al., 2015; Negoias et al., 2017; Pellegrino et al., 2019). Most of the studies reviewed in this article used the Sniffin’ Sticks test for olfactory assessment and otherwise conformed to the above parameters (Hummel et al., 1997).
Results
From this systematic review, the 18 studies reviewed are summarized in Appendix 1 and displayed in Tables 2 and 3. The detailed article summarizes are categorized in chronological order (oldest to recent) and by outcome variable of OT: 1) cognitive studies, 2) neuroimaging studies, 3) cognitive and neuroimaging studies, and 4) other.
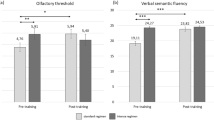
Cognition was assessed in five of the studies with limited sample sizes ranging from 33–91 participants, and with a limited cognitive battery, except for Cha et al. (2022) which had nine cognitive tests. Across all five studies, there is convergent findings that indicate OT in adults with and without olfactory loss at baseline can cognitively benefit from OT; albeit, for Chen et al. (2022), these results were mixed as the control group also experienced some cognitive improvements. Such cognitive benefit was observed in as little as 15 days in adults with dementia and as much as 6 months in healthy older adults. Cognitive benefits were observed in global cognition as well as the domains of verbal fluency, verbal learning and memory, and attention. In fact, four of these studies either had a no-contact control group or a more rigorous active (i.e., Suduko) control group or a sham OT group. Interestingly, a comparison in the dosage (2x/day versus 4x/day) suggests that a moderate dosage may be more effective than a higher dosage of OT. In general, the dosage of 2x/day of 4 or more odorants for 12 to 24 weeks was sufficient to produce these cognitive effects.
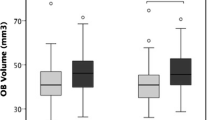
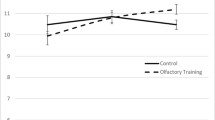
Neuroimaging was assessed in 13 of the studies that met the criteria for this systematic review. Most of the studies had small to moderate samples sizes (N’s = 7, 7, 11, 16, 25, 33, 36, 36, 37, 54, 58, 61, 97) for neuroimaging studies with a pre/post experimental design. First, four out of five studies that examined volume change in the olfactory bulb confirmed that OT (ranging from 16–24 weeks of OT dosage) resulted in an increased olfactory bulb volume, and this was observed across normosmic, hyposmic, and anosmic groups. Second, in the five studies that examined brain volume, all of them reported on increased volume after OT in several regions of interest including the cerebellum, thalamus, frontal cortex (i.e., right superior, right medial orbital), right gyrus rectus, right supplementary motor area, left precuneus, left superior medial cortex, left midcingulate cortex, hippocampus, and right insular, regions suggested to be involved with olfactory memory and verbal ability. These volume changes were observed after 6 weeks to 7 months of OT. Third, all six studies that examined brain connectivity confirmed that OT (ranging from 12–24 weeks of OT dosage) resulted in increased brain connectivity (or efficiency), and this was also observed across normosmic, hyposmic, and anosmic groups. In particular, such increased activation was observed in the dorsal anterior cingulate, several left hemisphere structures, orbitofrontal cortex, cingulate cortex to the insula, frontal lobe, and left parietal occipital juncture. Collectively, these neuroimaging studies indicated that various populations with or without olfactory loss experienced positive neuroplastic changes in the brain resulting from OT.
Beyond cognition and neuroimaging, only one study measured electro-physiological responses at the level of the olfactory epithelium. Hummel et al. (2018) observed in ansomics and hyposmics that OT was associated with a significantly higher number of electro-olfactogram responses.
Synthesis of Methodology of OT Studies
Methodologically, these OT studies have distinct and overlapping features that influence the quality of the data and the conclusions derived from them. Such features include the adequacy of control group(s), sample size and participant characteristics, treatment dosage, treatment adherence, length of follow-up, olfactory confounds, the inclusion of cognitive assessment and other neural measures.
Control Group Adequacy
Most (n = 10) of the reviewed studies used a one-group pre-post experimental design with no control group. The lack of a control group is considered a limitation as any type of participant engagement can exert an unknown influence on the dependent variables (i.e., cognition and brain changes). When a control group receives as much contact/engagement as the experimental group, the ability to derive causation improves. Yet the other eight studies had a variety of comparison groups such as comparing two OT dosages (Oleszkiewcz et al., 2022), comparing simple odorants to more complex mixture odorant OT (Oleszkiewcz et al., 2022), or comparing the OT to an active group (i.e., Sudoku group; Birte-Antina et al., 2018), or a standard no-contact control group (Oleszkiewcz et al., 2022). With OT in particular, developing an appropriate contact control condition remains a challenge. One could use non-scented stimuli as a sham condition, but the lack of an odorant may result in disinterest and poor adherence. However, Chen et al. (2022) did have an OT control group that was instructed to sniff bottles with no odorants. Even a control condition with only weak scents could be a confound as even an undetectable odor threshold could have an unknown impact on treatment outcomes as observed in Oleszkiewicz et al. (2021). Clearly, most of the reviewed articles were pilot studies with limited resources; thus, the choice of no active control group or no-contact control group was likely a financial one. The lack of an adequate control group represents a major criticism of this OT literature.
Sample Size and Attrition
As noted above, the reviewed OT studies appear to be predominantly pilot/feasibility studies; as such they were limited by small sample sizes that reduced their generalizability, power, and ecological validity. Albeit, many of these studies found a statistically significant therapeutic improvement in olfaction, cognition, and brain function, suggesting that these approaches are robust. Surprisingly, attrition was rarely reported, which is curious with a daily treatment protocol that requires 12–24 weeks to complete. Clearly, an intervention that requires engagement at least 2x/day would seem to be a burden that would affect attrition. Moving forward, the science of OT requires more rigor regarding treatment adherence, attrition, and larger sample sizes to ensure the generalizability of the findings, especially in different clinical populations that may have various olfactory and neurological risk factors.
Treatment Dosage
Dosage in OT studies is normally reported in the number of times per day participants are engaged in smelling the odorants over a period of time; typically, this is four odorants for 10–20 s each 2x/day (morning & evening) usually administered over 12–24 weeks. Oleszkiewicz et al. (2022) delved into the issue of dosage by comparing the effectiveness of training 2x/day versus 4x/day. Likewise, it is not clear in the OT literature presented why there is a focus on only four odorants; Oleszkiewicz et al. (2022) is the only OT where five odorants were used, and then replaced later with an additional five new odorants or in a separate study, Oleszkiewicz et al. (2022) used nine odorants in the OT. In fact, Altundag et al. (2015) modified OT by allowing participants to use three sets of four odorants sequentially after 12 and 24 weeks and found it to enhance the effectiveness of OT compared to the standard four odorants. Conceptually from a neuroplasticity perspective, it seems that providing more odorants would provide more novel stimulation that could improve olfaction and produce more robust cognitive outcomes (Vance et al., 2012).
Furthermore, since most of the studies with only 12 weeks of OT did not include cognitive measures, it is not clear whether this time frame is sufficient to produce a neuroplastic change reflective of cognitive improvement. However, olfactory bulb volume increases, structural volume increases, and functional connectivity changes were found after 12 weeks of training; this suggests that this time frame is sufficient to stimulate neuroplastic changes that could simultaneously support cognitive function.
Treatment Adherence
Most studies reported a diary or journal method to measure adherence. Unfortunately, few studies described this in detail and some studies failed to report their adherence data. Self-report of treatment adherence is subject to recall bias or social desirability. Some studies reported perfect adherence, but it seems unlikely from a practical standpoint that all participants were perfectly adherent to a protocol that requires twice daily engagement. Yet, since Al Aïn et al. (2019) conducted the OT in the laboratory with participants, they were the only ones to be able to have strong adherence data. Moving the OT literature forward, it is essential to apply more rigorous methods to quantify treatment adherence and incorporate adherence data into the data analysis. Without stronger adherence data, the findings of OT will be suspect, especially in being able to examine dosage responses.
Length of Follow-up
Although these studies do have substantial length of training of up to seven months, most lacked follow-up after training completion. Thus, it is not clear how robust the training effects on olfaction or cognitive/brain function outcomes would be following OT cessation. As a parallel example, in the ACTIVE Study, durability of the speed of processing training over 2–10 years was observed in sustained improvement of speed of processing and other areas (i.e., dementia risk, driving safety) (Edwards et al., 2017; Ross et al., 2016, 2017). Unfortunately, no such follow-up is available in these OT studies. Thus, it is important to document the durability of the OT effects as this determines its long-term effects for patients.
Olfactory Confounds
A potential confound that could impact both OT and olfactory assessment is exposure to other odorants in one’s environment. Eating aromatic foods, using colognes and scented hygiene products, lifestyle (i.e., smoking), and exposure to household odorants (i.e., incense, scented candles) could potentially impact one’s sense of smell which could impact the delivery of OT and olfactory assessment. Few studies control for this. For example, in a study of olfaction in 51 adults with HIV, Vance et al. (2020) specified in their appointment letter and in a checklist at the time of the study visit that participants should refrain from eating spicy or aromatic foods the night before and morning of their testing visit as this could interfere with the quality of the data. In our current systematic review, only one of the OT studies (i.e., Oleszkiewicz et al., 2022) specifically instructed participants not to engage in the OT 30 min before or after meals as this could potentially interfere with the intervention. Moving forward, to further the rigor in OT, it is essential to consider whether such natural daily exposures to odorants in the environment are negligible to OT or whether this is an important variable to control.
Cognitive Assessment and Neuroimaging Markers
Only a limited cognitive battery was administered when cognitive measures were included in these OT studies. The inclusion of a complete cognitive battery, representing a range of cognitive domains including attention, speed of processing, verbal learning and memory, visuospatial learning and memory, and executive functioning, would allow a more comprehensive examination of the impact of OT on cognitive functioning. It is likely that OT could potentially impact several cognitive domains.
Furthermore, only one of the reviewed OT studies included both cognitive and neuroimaging measures (Chen et al., 2022). Thus, it is difficult to determine the corresponding neural correlates of OT-induced cognitive improvement. The rigor of future studies could be substantially improved by assessing cognition while acquiring multimodal neurophysiological data (structural MRI, fMRI, diffusion tensor imaging, EEG/ERP, neuronal biomarkers) in the same participants. In fact, as cognitive training and other types of cognitive rehabilitation has been shown to increase neuronal biomarkers such as Brain-Derived Neurotropic Factor (e.g., Angelucci et al., 2015), such markers of neurological improvement may be salient in OT studies.
Discussion
In our review of OT studies, we found that OT improved olfaction over baseline performance in those with and without olfaction loss. In general, this olfactory improvement was associated with improved cognition and changes in neurological structures and connections. Specifically, these changes included: 1) improved cognition (i.e., verbal fluency, memory, global cognition Birte-Antina et al., 2018; Knudsen et al., 2015; Oleszkiewicz et al., 2021; Oleszkiewicz et al., 2022); 2) increased olfactory bulb volume (Gellrich et al., 2018; Mahmut et al., 2020; Negoias et al., 2017); 3) increased volume in hippocampal, cerebellum, and thalamic regions (Gellrich et al., 2018); 4) increased neural signal activity (Kollndorfer et al., 2015); 5) greater functional connectivity in the chemosensory processing networks (Kollndorfer et al., 2015); 6) new activation in the right dorsal anterior cingulate (Pellegrino et al., 2019); 7) increased activation in several left frontal areas associated with language (Pellegrino et al., 2019); and 8) increased response amplitude of the olfactory epithelium (Hummel et al., 2018)..
Theories of OT and Cognition
In the context of olfactory loss, prior research has found grey matter volume decreases in the anterior cingulate cortex and insula, as well as the cerebellum (Bitter et al., 2010; Reichert & Schöpf, 2018). The anterior cingulate cortex is associated with executive functioning (Devinsky et al., 1995), particularly conflict monitoring (Botvinick et al., 2004). In addition to being linked with the gustatory cortex, the insula has also been associated with emotion and cognition (Gasquoine, 2014) though the integration of different functional systems that are involved in sensory-motor processing, affect, and cognition, including language and attention (Chang et al., 2013; Uddin et al., 2017). Moreover, both the anterior cingulate cortex and insula have been associated with olfaction in fMRI studies and increased blood-oxygen-level-dependent (BOLD) activation has been observed in the cerebellum in response to olfactory stimulation (Albrecht et al., 2010; Ferdon & Murphy, 2003; Savic, 2002; Wabnegger & Schienle, 2019). The cerebellum is involved in more than just motor control, with the posterior lobe of the cerebellum linked with numerous cognitive functions, including working memory, planning/organization, strategy development, verbal fluency, and error awareness (Schmahmann, 2019). Individuals with complete anosmia also show less activation in the dorsolateral prefrontal cortex (Iannilli et al., 2011), a region associated with cognitive control and working memory (Andrews et al., 2011; MacDonald et al., 2000). Additionally, there is a direct link between olfactory processing and the hippocampus and entorhinal cortex (Biella & De Curtis, 2000; Kubota et al., 2020; Rai et al., 2021; Vanderwolf, 1992). Indeed, it has been hypothesized that disruption of olfactory-entorhinal cortex-hippocampus circuitry upregulates memory decline (Daulatzai, 2015).
With these associations of olfactory loss and functional and structural neural correlates in regions associated with attention, language, memory, and higher-order executive functioning, it is not surprising that olfactory and cognitive declines are associated. Moreover, the link between neurological illnesses (i.e., Parkinson’s disease, Alzheimer’s dementia, frontotemporal dementia, & epilepsy) and anosmia also makes conceptual sense (Doty, 2012; Kamath et al., 2019; Khurshid et al., 2019; Kulason et al., 2021).
Is it possible to improve olfaction, cognition, and neural processing though OT? Regarding the link between olfaction and grey matter volume, there is growing evidence that OT not only improves olfaction but also yields increased grey matter volume in multiple critical regions such as the hippocampus and entorhinal cortex, inferior, middle, and superior frontal gyri, and the cerebellum (Al Aïn et al., 2019; Rezaeyan et al., 2022). While there is a paucity of research examining how cognition is affected by OT, based on these MRI volume findings, we can hypothesize that attention, memory, and executive functioning abilities should improve post-OT. Pulling from the traumatic brain injury literature, the spontaneous return of olfactory functioning, as well as improved olfaction following OT, has been associated with both an increase in olfactory bulb volume, attributed to increased glomerular dopaminergic interneurons, as well as increased subventricular neurogenesis (Marin et al., 2020). There appears to be lifelong neurogenesis in the subventricular zone, which is found in the lateral ventricles, and in adults, this migrates anteriorly into the olfactory bulb (Lim & Alvarez-Buylla, 2016). It is possible that OT stimulates the subventricular zone, thus increasing the number of interneurons and the olfactory bulb volume. Given the afferent and efferent projections between the olfactory bulb and the above-mentioned neuroanatomical regions, neuroplastic changes likely result in increased grey matter volume in multiple brain regions. We speculate that this will then translate to improved cognitive functioning.
Implications for Clinical Practice
Prior studies and systematic reviews indicate that OT is safe and can be used to improve olfaction in those with and without olfactory loss (Doty, 2019; Patel, 2017). Our systematic review provides further evidence that OT may provide both cognitive and neurological benefits. Given the inexpensive nature and safety of OT, clinicians can suggest its use to patients interested in trying this on their own. In fact, in participants with idiopathic and post-infectious olfactory loss, Patel et al. (2017) observed that OT that used random concentrations of essential oils was just as effective as standard OT with control concentrations; perhaps there would be a cognitive or neurological benefit as well. Albeit, patients should be warned that the efficacy of OT on cognitive and neurological outcomes has not been firmly established, nor has the type of delivery protocol, types of odorants, or dosage of OT been clinically defined to produce optimal therapeutic benefit. Thus, we assert that this article is not an endorsement for people to buy essential oils or other odorants with the expectation that it is a “cure all” for everything from improved sensory acuity to preventing Alzheimer’s disease. Substantially more research in both healthy and various clinical populations is needed before OT can be empirically supported.
Implications for Research
There are several implications from this systematic review which provide future directions on OT as a potential neurocognitive therapeutic tool, many of which are already highlighted in the synthesis section above. First, to more comprehensively quantify olfaction, studies should measure odor identification, odor detection threshold (below – Sniff Magnitude Test), and odor discrimination, and perhaps measures of olfactory-related ERPs which can provide more specific physiological data to olfaction (Gudziol & Guntinas-Lichius, 2019).
Second, fMRI and structural MRI should continue to be included in research designs to further expand our knowledge of the underlying neural mechanisms. While functional MRI can provide substantial information about the spatial resolution of neural processing and complement the high temporal resolution of olfactory-related ERPs, these measures currently lack clinical correlations in this literature. A task-based fMRI study can indicate that a specific cognitive ability is aberrant relative to a control group, but it is difficult to directly translate this to clinical practice. Therefore, neuroimaging and OT studies should also include baseline and post-training cognitive assessments. The direct measure of cognitive functioning will expand our understanding of underlying mechanisms and will help determine the added benefit of OT beyond improved olfaction.
Third, OT could be improved through applying methods from the multisensory literature. For example, speech perception in a noisy environment is improved in both healthy and clinical populations when auditory-verbal stimuli are paired together, rather than auditory-alone or visual-alone (Foxe et al., 2015; Ross et al., 2007). A similar approach can be taken with paired olfactory and visual sensory inputs. In an animal model targeting both the gustatory cortex and piriform olfactory cortex, there is evidence of multisensory integration, with optical inhibition of gustatory cortex neurons resulting in aberrant recognition of odor stimuli (Maier et al., 2015). In humans, visual objects that are associated with an odor are processed in the posterior piriform cortex, with activation in this region increasing when, more senses are providing congruent information (Porada et al., 2019), with both studies suggesting the piriform cortex is a crucial node in the olfactory multisensory network. Leveraging this multisensory relationship between olfaction and visual stimulation in OT protocols could potentially enhance efficacy on olfactory and cognitive outcomes.
Fourth, sex differences in olfaction have not been directly examined in OT but could be a useful variable to consider in future studies. In particular, a female advantage of olfaction has been attributed to the indirect influence of gonadal hormones, menstrual cycle-related fluctuations, and neuroendocrine influences on brain regions involved in olfactory processing (Doty & Cameron, 2009; Sorokowski et al., 2019). Social factors can also contribute as women generally experience greater olfactory awareness, odor familiarity, and greater exposure to odors in their social environment. In contrast, men experience a greater risk of toxic exposure to chemicals and hazards in their occupational environments. As odor identification tasks require assigning a verbal label to a retrieved odor memory, the enhanced verbal abilities observed in women may also explain these differences (Doty & Cameron, 2009; Sorokowski et al., 2019).
Fifth, as OT was conducted in older adults, healthy younger adults, those with traumatic brain injury, and with various levels of olfaction from normosmic to anosmic, this may be an intervention in other clinical populations vulnerable to both cognitive and olfactory deficits such as those with HIV, mild cognitive impairment, early-stage Alzheimer’s disease, and Parkinson’s disease (Vance & Brew, 2021). Due to the COVID-19 pandemic, chronic olfactory loss is a major growing public health concern, with consequences of anosmia including poor quality of life, inadequate nutritional intake, and increased risk of psychiatric conditions. It has recently been estimated that 700 K to 1.6 M persons living in the US will endure long-term smell dysfunction due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Khan et al., 2022). As COVID-19 is associated with deficits in olfaction and cognition, OT may be a potential therapeutic technique that addresses both olfaction and cognitive dysfunction (Vance et al., 2021).
Sixth, more study is needed to investigate the bottom-up and top-down processes involved with OT. These OT studies generally support the idea that OT, which involves stimulating olfactory epithelium and olfactory receptors peripherally, improves cognition and brain function; this represents a bottom-up process. Albeit, Negoias et al. (2017) observed that lateralized OT to either the right or left nostril of healthy people resulted in an olfactory bulb volume increase and improved olfactory detection thresholds in the untrained nostril; this indicates a top-down process. Future studies may also investigate whether improving cognition, via cognitive training or other such intervention methods, produces a top-down improvement in olfaction as well.
Seventh, another mechanism by which OT could improve cognition and brain function is through the enhancement of mood. For example, Birte-Antina et al. (2018) found that OT decreased depression. Odorants have been reported to enhance mood in general (Chen & Chen, 2015; Doty, 2019). Given the connection between mood, cognition, and brain function (Doty, 2019; Uddin et al., 2017), one hypothesis is that OT could improve mood which downstream improves cognition. Another possible pathway is through the insula, which is involved in cognitive processing and affect, and is also related to olfaction (Chang et al., 2013; Uddin et al., 2017). Diminished olfactory abilities in major depression are accompanied by corresponding changes in the peripheral and central olfactory system, including abnormalities in olfactory event-related potentials (OERPs), olfactory sulcal depth, olfactory bulb volume, and olfactory fMRI (Croy and Hummel, 2017; Negoias, et al., 2010; Pause et al., 2003; Rottstaedt et al., 2018a, b; Takahashi et al., 2016). Interestingly, performance on olfactory psychophysical testing, fMRI BOLD response to odorants, and OERPs improved with treatment in the form of psychotherapy and medication (Croy et al., 2017; Pause et al., 2003).
Eighth, if we can improve the effectiveness of OT, we may also be able to improve its cognitive and neurological impact. The literature suggests that an underlying inflammation of the paranasal sinus epithelium may be an unrecognized contributor to olfactory impairment, such that, treating sinonasal inflammatiion may also improve OT outcomes. Nguyen and Patel (2018) randomized 133 hyposmic patients without sinonasal inflammation into two groups. The experimental group received standard OT plus daily 2x/day self-administered nasal irrigation with budesonide, a steroid that reduces inflammation. The control group also received standard OT but was irrigated with saline. Analysis revealed that 43.9% of the experimental group (OT + budesonide irrigation) and 26.9% of the control group (OT + saline irrigation) experienced a clinically significant change in smell identification (i.e., UPSIT score; OR = 3.93, 95% CI 1.20–12.88). Given this dramatic boost to OT effectiveness, it is worth examining if this extra OT benefit due to budesonide irrigation would produce a larger cognitive and neurological benefit as well.
Ninth, the psychophysics of odorants should also be considered in future studies. Presumably, the molecular shape, size, or complexity of odorants may produce various benefits on olfactory receptors, creating differential effects on olfaction and cognition. This area is touched upon by the Oleszkiewicz et al.’s (2021) study that examined the impact of “simple” single molecule OT vs more complex multi-molecular odorant mixtures OT on cognition; more complex odorants did not produce greater cognitive or olfactory outcomes. Similarly, Poletti et al. (2017) randomized a sample of 98 adults with posttraumatic and post-viral olfactory loss to receive OT with either five months of light weight molecule odorants (LWM; < 150 g/mol) OT or heavy weight molecule odorants (HWM; > 150 g/mol) OT. Researchers concluded that olfaction improved similarly in both OT groups, with the exception that HWM OT corresponded to a greater odorant threshold improvement in the posttraumatic olfactory loss participants. Similarly, Sinding et al. (2014) examined age-related olfactory sensitivity for light (< 150 g/mol) vs heavy (> 150 g/mol) molecules in normosmic younger and older adults. Researchers found that younger adults were sensitive to both light and heavy molecular odorants but older adults were less sensitive to heavy molecule odorants. This suggests that OT in older adults may require the use of light molecule odorants. This becomes more complex when we consider that with age, people are less sensitive to detect pleasant odors while retaining sensitivity to unpleasant odors (Khan et al., 2007). Moreover, research also shows that the structure of the odor molecular can produce different odorants (e.g., lactones = apricot/coconut smell; volatile fatty acids = rancid/sour smell; esters = fruity smell) (Genva et al., 2019). With these caveats, studies should consider reporting the molecular information of odorants so that OT can eventually be standardized and patterns can be detected in the literature.
A limitation of this systematic review is that we did not conduct a meta-analysis; however, there were several reasons that would have diminished the scientific value of such a meta-analysis at this time. First, the data reflect heterogenous OTs making it difficult, if not impossible, to pool. OTs vary widely by: a) treatment lengths (i.e., days – months), b) amount engaged in each odorant (i.e., sniffing the odorants from 5 s – 30 s each); c) times per day to smell odorants (i.e., not specified, 2x/day, 4x/day); d) number of odorants (i.e., 1 odorant – 40 odorants), and e) types of odorants (i.e., n-butanol, lemon, phenethylamine, simple odorants vs complex odorants). Second, studies included a wide variety of clinical populations (i.e., healthy adults, those with dementia, men with laryngectomy, younger and older adults) and with a range of olfactory abilities (i.e., normosmia, anosmia, hyposmia). Both considerations are problematic because it is unclear how to factor in the effect OT truly has as the effect sizes would vary drastically based on type of population and olfactory ability level. Third, the cognitive/neurological outcomes of OT also vary dramatically as it is not clear how to standardize and combine the neuroimaging and cognitive data into a composite that reflects a single neurological outcome coefficient that can be reliably and validly incorporated into a meta-analysis. Even with just the cognitive data, some studies simply employ a cognitive screener (e.g., MOCA) while others may have a partial or full cognitive battery; and even when certain cognitive domains (e.g., executive function) may be represented in certain studies, the cognitive measures are different. Fourth, treatment fidelity was poorly measured or non-existent in most studies. Thus, it is unclear how much OT participants received. Given these limitations in the OT literature, generating an effect size of OT on neurocognition is not warranted at this time as a statistical value from such a meta-analysis may mischaracterize the true effect. Future studies of OT need to address many of these methodological limitations so that eventually this connection of OT on neurocognitive outcomes, as well as the mediation effect of improved olfaction on such neurocognitive outcomes, be examined with better scientific rigor.
Finally, a limitation of this systematic review is that we did not incorporate all interventions that could improve olfaction. For example, based on a case study, photobiomodulation therapy (i.e., light helmet, body pad, intranasal piece) has been suggested as a way to improve olfaction and cognition (Salehpour et al., 2019). Focusing on OT exclusively in this review was strategic as other types of olfactory intervention may not be as simple to administer as OT, which is also inexpensive and seems face-valid and readily understood by people.
Conclusion
Although not conclusive, the reviewed OT studies on cognition and neuroimaging provided converging evidence that OT improved brain function as exhibited by improved cognitive performance, increased volumes in several brain regions, and increased neural connectivity/efficiency. Albeit, with such small sample sizes, the summary from this systematic review should be drawn cautiously, as sample error and lack of multiple comparison corrections could have played a role in our conclusions. More rigorous research is needed as these studies suffer from small sample sizes which limit generalizability, lack active control groups needed for proper experimental comparisons, used limited cognitive measures to determine their impact across a broad range of cognitive domains, and lack significant long-term follow-up assessment needed to determine robustness of training effects are over time. Despite these limitations, the converging evidence is compelling, clearly showing the potential of OT to improve cognitive and brain function in a range of adults with and without olfactory loss.
Data Availability
This is a systematic review. We have provided the search parameters and mesh terms (Table 1) whereby others can replicate the search and reproduce the findings. Actual tabulation of the reviewed articles and summaries of the articles are provided in this systematic review in Tables 2 and 3 and the appendix.
References
Adams, D. R., Kern, D. W., Wroblewski, K. E., McClintock, M. K., Dale, W., & Pinto, J. M. (2018). Olfactory dysfunction predicts subsequent dementia in older U.S. adults. Journal of the American Geriatrics Society, 66(1), 140–144. https://doi.org/10.1111/jgs.15048
Al Aïn, S., Poupon, D., Hétu, S., Mercier, N., Steffener, J., & Frasnelli, J. (2019). Smell training improves olfactory function and alters brain structure. NeuroImage, 189, 45–54. https://doi.org/10.1016/j.neuroimage.2019.01.008
Albrecht, J., Kopietz, R., Frasnelli, J., Wiesmann, M., Hummel, T., & Lundström, J. N. (2010). The neuronal correlates of intranasal trigeminal function-an ALE meta-analysis of human functional brain imaging data. Brain Research Reviews, 62(2), 183–196. https://doi.org/10.1016/j.brainresrev.2009.11.001
Altundag, A., Cayonu, M., Kayabasoglu, G., Salihoglu, M., Tekeli, H., Saglam, O., & Hummel, T. (2015). Modified olfactory training in patients with postinfectious olfactory loss. The Laryngoscope, 125(8), 1763–1766. https://doi.org/10.1002/lary.25245
Andrews, S. C., Hoy, K. E., Enticott, P. G., Daskalakis, Z. J., & Fitzgerald, P. B. (2011). Improving working memory: The effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimulation, 4(2), 84–89. https://doi.org/10.1016/j.brs.2010.06.004
Angelucci, F., Peppe, A., Carlesimo, G. A., Serafini, F., Zabberoni, S., Barban, F., Shofany, J., Caltagirone, C., & Costa, A. (2015). A pilot study on the effect of cognitive training on BDNF serum levels in individuals with Parkinson’s disease. Frontiers in Human Neuroscience, 9, 130. https://doi.org/10.3389/fnhum.2015.00130
Bialystok, C. F. I., Klein, R., & Viswanathan, M. (2004). Bilingualism, aging, and cognitive control: Evidence from the Simon task. Psychology and Aging, 19(2), 290–303. https://doi.org/10.1037/0882-7974.19.2.290
Biella, G., & de Curtis, M. (2000). Olfactory inputs activate the medial entorhinal cortex via the hippocampus. Journal of Neurophysiology, 83(4), 1924–1931. https://doi.org/10.1152/jn.2000.83.4.1924
Birte-Antina, W., Ilona, C., Antje, H., & Thomas, H. (2018). Olfactory training with older people. International Journal of Geriatric Psychiatry, 33(1), 212–220. https://doi.org/10.1002/gps.4725
Bitter, T., Brüderle, J., Gudziol, H., Burmeister, H. P., Gaser, C., & Guntinas-Lichius, O. (2010). Gray and white matter reduction in hyposmic subjects—A voxel-based morphometry study. Brain Research, 1347, 42–47. https://doi.org/10.1016/j.brainres.2010.06.003
Botvinick, M. M., Cohen, J. D., & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. https://doi.org/10.1016/j.tics.2004.10.003
Brewster, K. K., Hu, M. C., Wall, M. M., Brown, P. J., Zilcha-Mano, S., Roose, S. P., Stein, A., Golub, J. S., & Rutherford, B. R. (2021). Age-Related Hearing Loss, Neuropsychological Performance, and Incident Dementia in Older Adults. Journal of Alzheimer's disease: JAD, 80(2), 855–864. https://doi.org/10.3233/JAD-200908
Carr, D. C., Willis, R., Kail, B. L., & Carstensen, L. L. (2020). Alternative retirement paths and cognitive performance: Exploring the role of preretirement job complexity. The Gerontologist, 60(3), 460–471. https://doi.org/10.1093/geront/gnz079
Cha, H., Kim, S., Kim, H., Kim, G., & Kwon, K. Y. (2022). Effect of intensive olfactory training for cognitive function in patients with dementia. Geriatrics & Gerontology International, 22(1), 5–11. https://doi.org/10.1111/ggi.14287
Chang, L. J., Yarkoni, T., Khaw, M. W., & Sanfey, A. G. (2013). Decoding the role of the insula in human cognition: Functional parcellation and large-scale reverse inference. Cerebral Cortex, 23(3), 739–749. https://doi.org/10.1093/cercor/bhs065
Chen, S. L., & Chen, C. H. (2015). Effects of lavender tea on fatigue, depression, and maternal-infant attachment in sleep-disturbed postnatal women. Worldviews on Evidence-Based Nursing, 12(6), 370–379. https://doi.org/10.1111/wvn.12122
Chen, B., Espin, M., Haussmann, R., Matthes, C., Donix, M., Hummel, T., & Haehner, A. (2022). The effect of olfactory training on olfaction, cognition, and brain function in patients with mild cognitive impairment. Journal of Alzheimer’s Disease, 85(2), 745–754. https://doi.org/10.3233/JAD-215257
Croy, I., & Hummel, T. (2017). Olfaction as a marker for depression. Journal of Neurology, 264(4), 631–638. https://doi.org/10.1007/s00415-016-8227-8
Daulatzai, M. A. (2015). Olfactory dysfunction: Its early temporal relationship and neural correlates in the pathogenesis of Alzheimer’s disease. Journal of Neural Transmission, 122(10), 1475–1497. https://doi.org/10.1007/s00702-015-1404-6
Devanand, D. P. (2016). Olfactory identification deficits, cognitive decline, and dementia in older adults. American Journal of Geriatric Psychiatry, 24(12), 1151–1157. https://doi.org/10.1016/j.jagp.2016.08.010
Devanand, D. P., Lee, S., Manly, J., Andrews, H., Schupf, N., Doty, R. L., Stern, Y., Zahodne, L. B., Louis, E. D., & Mayeux, R. (2015). Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology, 84(2), 182–189. https://doi.org/10.1212/WNL.0000000000001132
Devinsky, O., Morrell, M. J., & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain: A Journal of Neurology, 118(Pt 1), 279–306. https://doi.org/10.1093/brain/118.1.279
Doty, R. L. (2012). Olfactory dysfunction in Parkinson disease. Nature Reviews. Neurology, 8(6), 329–339. https://doi.org/10.1038/nrneurol.2012.80
Doty, R. L. (2019). Treatments for smell and taste disorders: A critical review. Handbook of Clinical Neurology, 164, 455–479. https://doi.org/10.1016/B978-0-444-63855-7.00025-3
Doty, R. L., & Cameron, E. L. (2009). Sex differences and reproductive hormone influences on human odor perception. Physiology & Behavior, 97(2), 213–228. https://doi.org/10.1016/j.physbeh.2009.02.032
Dulay, M. F., & Murphy, C. (2002). Olfactory acuity and cognitive function converge in older adulthood: Support for the common cause hypothesis. Psychology and Aging, 17(3), 392–404.
Edwards, J. D., Xu, H., Clark, D. O., Guey, L. T., Ross, L. A., & Unverzagt, F. W. (2017). Speed of processing training results in lower risk of dementia. Alzheimer’s and Dementia, 3(4), 603–611. https://doi.org/10.1016/j.trci.2017.09.002
Eliyan, Y., Wroblewski, K. E., McClintock, M. K., & Pinto, J. M. (2020). Olfactory dysfunction predicts the development of depression in older US adults. Chemical Senses, 46, bjaa075. https://doi.org/10.1093/chemse/bjaa075
Ferdon, S., & Murphy, C. (2003). The cerebellum and olfaction in the aging brain: A functional magnetic resonance imaging study. NeuroImage, 20(1), 12–21. https://doi.org/10.1016/s1053-8119(03)00276-3
Foxe, J. J., Molholm, S., Del Bene, V. A., Frey, H. P., Russo, N. N., Blanco, D., Saint-Amour, D., & Ross, L. A. (2015). Severe multisensory speech integration deficits in high-functioning school-aged children with Autism Spectrum Disorder (ASD) and their resolution during early adolescence. Cerebral Cortex, 25(2), 298–312. https://doi.org/10.1093/cercor/bht213
Gasquoine, P. G. (2014). Contributions of the insula to cognition and emotion. Neuropsychology Review, 24(2), 77–87. https://doi.org/10.1007/s11065-014-9246-9
Gellrich, J., Han, P., Manesse, C., Betz, A., Junghanns, A., Raue, C., Schriever, V. A., & Hummel, T. (2018). Brain volume changes in hyposmic patients before and after olfactory training. The Laryngoscope, 128(7), 1531–1536. https://doi.org/10.1002/lary.27045
Genva, M., Kenne Kemene, T., Deleu, M., Lins, L., & Fauconnier, M. L. (2019). Is it possible to predict the odor of a molecule on the basis of its structure? International Journal of Molecular Sciences, 20(12), 3018. https://doi.org/10.3390/ijms20123018
Gudziol, H., & Guntinas-Lichius, O. (2019). Electrophysiologic assessment of olfactory and gustatory function. Handbook of Clinical Neurology, 164, 247–262. https://doi.org/10.1016/B978-0-444-63855-7.00016-2
Gürbüz, D., Kesimli, M. C., Bilgili, A. M., & Durmaz, H. Ö. (2022). Olfactory rehabilitation and olfactory lobe volume changes in patients after total laryngectomy: A prospective randomized study. Brazilian Journal of Otorhinolaryngology, 88(4), 607–612. https://doi.org/10.1016/j.bjorl.2021.02.013
Han, P., Musch, M., Abolmaali, N., & Hummel, T. (2021). Improved odor identification ability and increased regional gray matter volume after olfactory training in patients with idiopathic olfactory loss. i-Perception, 12(2), 20416695211005812. https://doi.org/10.1177/20416695211005811
Hosseini, K., Zare-Sadeghi, A., Sadigh-Eteghad, S., Mirsalehi, M., & Khezerloo, D. (2020). Effects of olfactory training on resting-state effective connectivity in patients with posttraumatic olfactory dysfunction. Acta Neurobiologiae Experimentalis, 80(4), 381–388. https://doi.org/10.21307/ane-2020-035
Hummel, T., Sekinger, B., Wolf, S. R., Pauli, E., & Kobal, G. (1997). “Sniffin” sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chemical Senses, 22(1), 39–52. https://doi.org/10.1093/chemse/22.1.39
Hummel, T., Stupka, G., Haehner, A., & Poletti, S. C. (2018). Olfactory training changes electrophysiological responses at the level of the olfactory epithelium. Rhinology, 56(4), 330–335. https://doi.org/10.4193/Rhin17.163
Iannilli, E., Bitter, T., Gudziol, H., Burmeister, H. P., Mentzel, H. J., Chopra, A. P., & Hummel, T. (2011). Differences in anosmic and normosmic group in bimodal odorant perception: A functional- MRI study. Rhinology, 49(4), 458–463. https://doi.org/10.4193/Rhino11.110
Jiramongkolchai, P., Jones, M. S., Peterson, A., Lee, J. J., Liebendorfer, A., Klatt-Cromwell, C. N., Schneider, J. S., Drescher, A. J., Ogden, M. A., Brunworth, J. D., Kallogjeri, D., Kukuljan, S., Peelle, J. E., & Piccirillo, J. F. (2021). Association of olfactory training with neural connectivity in adults with postviral olfactory dysfunction. JAMA Otolaryngology- Head & Neck Surgery, 147(6), 502–509. https://doi.org/10.1001/jamaoto.2021.0086
Johnson A. J. (2011). Cognitive facilitation following intentional odor exposure. Sensors (Basel, Switzerland), 11(5), 5469–5488. https://doi.org/10.3390/s110505469
Jung, H. J., Shin, I. S., & Lee, J. E. (2019). Olfactory function in mild cognitive impairment and Alzhiemer’s disease: A meta-analysis. The Laryngoscope, 129(2), 362–369. https://doi.org/10.1002/lary.27399
Kamath, V., Chaney, G. S., DeRight, J., & Onyike, C. U. (2019). A meta-analysis of neuropsychological, social cognitive, and olfactory functioning in the behavioral and language variants of frontotemporal dementia. Psychological Medicine, 49(16), 2669–2680. https://doi.org/10.1017/S0033291718003604
Khan, R. M., Luk, C. H., Flinker, A., Aggarwal, A., Lapid, H., Haddad, R., & Sobel, N. (2007). Predicting odor pleasantness from odorant structure: Pleasantness as a reflection of the physical world. The Journal of Neuroscience, 27(37), 10015–10023. https://doi.org/10.1523/JNEUROSCI.1158-07.2007
Khan, A. M., Kallogjeri, D., & Piccirillo, J. F. (2022). Growing public health concern of COVID-19 chronic olfactory dysfunction. JAMA Otolaryngology - Head & Neck Surgery, 148(1), 81–82. https://doi.org/10.1001/jamaoto.2021.3379
Kheirkhah, F., Roustaei, G., Mohebbi Abivardi, E., Hamidia, A., & Javadian Kutenai, S. (2018). Improvement in Cognitive Status and Depressive Symptoms Three Months after Cataract Surgery. Caspian Journal of Internal Medicine, 9(4), 386–392. https://doi.org/10.22088/cjim.9.4.386
Khurshid, K., Crow, A., Rupert, P. E., Minniti, N. L., Carswell, M. A., Mechanic-Hamilton, D. J., Kamath, V., Doty, R. L., Moberg, P. J., & Roalf, D. R. (2019). A quantitative meta-analysis of olfactory dysfunction in epilepsy. Neuropsychology Review, 29(3), 328–337. https://doi.org/10.1007/s11065-019-09406-7
Knopke, S., & Olze, H. (2018). Hörrehabilitation mithilfe von Cochleaimplantaten und kognitive Fähigkeiten [Hearing rehabilitation with cochlear implants and cognitive abilities]. HNO, 66(5), 364–368. https://doi.org/10.1007/s00106-017-0423-z
Knudsen, K., Flensborg Damholdt, M., Mouridsen, K., & Borghammer, P. (2015). Olfactory function in Parkinson’s Disease - effects of training. Acta Neurologica Scandinavica, 132(6), 395–400. https://doi.org/10.1111/ane.12406
Kollndorfer, K., Kowalczyk, K., Hoche, E., Mueller, C. A., Pollak, M., Trattnig, S., & Schöpf, V. (2014). Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plasticity, 2014, 140419. https://doi.org/10.1155/2014/140419
Kollndorfer, K., Fischmeister, F. P., Kowalczyk, K., Hoche, E., Mueller, C. A., Trattnig, S., & Schöpf, V. (2015). Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. NeuroImage: Clinical, 9, 401–410. https://doi.org/10.1016/j.nicl.2015.09.004. PMID: 26594622; PMCID: PMC4590718.
Kubota, S., Masaoka, Y., Sugiyama, H., Yoshida, M., Yoshikawa, A., Koiwa, N., Honma, M., Kinno, R., Watanabe, K., Iizuka, N., Ida, M., Ono, K., & Izumizaki, M. (2020). Hippocampus and parahippocampus volume reduction associated with impaired olfactory abilities in subjects without evidence of cognitive decline. Frontiers in Human Neuroscience, 14, 556519. https://doi.org/10.3389/fnhum.2020.556519
Kuhl, P. K., Corrigan, N. M., van den Bosch, J. J. F., Can, D. O., & Richards, T. (2016). Neuroimaging of the bilingual brain: Structural brain correlates of listening and speaking. Brain and Language, 162, 1–9. https://doi.org/10.1016/jbandl.2016.07.004
Kulason, S., Ratnanather, J. T., Miller, M. I., Kamath, V., Hua, J., Yang, K., Ma, M., Ishizuka, K., & Sawa, A. (2021). A comparative neuroimaging perspective of olfaction and higher-order olfactory processing: On health and disease. Seminars in Cell & Developmental Biology, S1084–9521(21)00221–4. Advance online publication. https://doi.org/10.1016/j.semcdb.2021.08.009
Lampit, A., Hallock, H., & Valenzuela, M. (2014). Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Medicine, 11(11), e1001756. https://doi.org/10.1371/journal.pmed.1001756
Leon, M., & Woo, C. (2018). Environmental enrichment and successful aging. Frontiers in Behavioral Neuroscience, 12, 155. https://doi.org/10.3389/fnbeh.2018.00155
Lim, D. A., & Alvarez-Buylla, A. (2016). The adult ventricular-subventricular zone (V-SVZ) and OB (OB) neurogenesis. Cold Spring Harbor Perspectives in Biology, 8(5), a018820. https://doi.org/10.1101/cshperspect.a018820
Livermore, A., & Laing, D. G. (1996). Influence of training and experience on the perception of multicomponent odor mixtures. Journal of Experimental Psychology: Human Perception and Performance, 22(2), 267–277. https://doi.org/10.1037//0096-1523.22.2.267
MacDonald, A. W., 3rd., Cohen, J. D., Stenger, V. A., & Carter, C. S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. https://doi.org/10.1126/science.288.5472.1835
MacDonald, S., Keller, C., Brewster, P., & Dixon, R. A. (2018). Contrasting olfaction, vision, and audition as predictors of cognitive change and impairment in non-demented older adults. Neuropsychology, 32(4), 450–460. https://doi.org/10.1037/neu0000439
Mahmut, M. K., Musch, M., Han, P., Abolmaali, N., & Hummel, T. (2020). The effect of olfactory training on OB volumes in patients with idiopathic olfactory loss. Rhinology, 58(4), 410–412. https://doi.org/10.4193/Rhin20.223
Maier, J. X., Blankenship, M. L., Li, J. X., & Katz, D. B. (2015). A multisensory network for olfactory processing. Current Biology, 25(20), 2642–2650. https://doi.org/10.1016/j.cub.2015.08.060
Marin, C., Langdon, C., Alobid, I., & Mullol, J. (2020). Olfactory dysfunction in traumatic brain injury: The role of neurogenesis. Current Allergy and Asthma Reports, 20(10), 55. https://doi.org/10.1007/s11882-020-00949-x
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine, 6(7), e1000097. https://doi.org/10.1371/journal.pmed.1000097
Negoias, S., Croy, I., Gerber, J., Puschmann, S., Petrowski, K., Joraschky, P., & Hummel, T. (2010). Reduced OB volume and olfactory sensitivity in patients with acute major depression. Neuroscience, 169(1), 415–421. https://doi.org/10.1016/j.neuroscience.2010.05.012
Negoias, S., Pietsch, K., & Hummel, T. (2017). Changes in OB volume following lateralized olfactory training. Brain Imaging and Behavior, 11(4), 998–1005. https://doi.org/10.1007/s11682-016-9567-9
Nguyen, T. P., & Patel, Z. M. (2018). Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. International Forum of Allergy and Rhinology, 8(9), 977–981. https://doi.org/10.1002/alr.22140
Oleszkiewicz, A., Abriat, A., Doelz, G., Azema, E., & Hummel, T. (2021). Beyond olfaction: Beneficial effects of olfactory training extend to aging-related cognitive decline. Behavioral Neuroscience, 135(6), 732–740. https://doi.org/10.1037/bne0000478
Oleszkiewicz, A., Bottesi, L., Pieniak, M., Fujita, S., Krasteva, N., Nelles, G., & Hummel, T. (2022). Olfactory training with aromastics: Olfactory and cognitive effects. European Archives of Oto-Rhino-Laryngology, 279(1), 225–232. https://doi.org/10.1007/s00405-021-06810-9
Patel, Z. M. (2017). The evidence for olfactory training in treating patients with olfactory loss. Current Opinion in Otolaryngology & Head and Neck Surgery, 25(1), 43–46. https://doi.org/10.1097/MOO.0000000000000328
Patel, Z. M., Wise, S. K., & DelGaudio, J. M. (2017). Randomized controlled trial demonstrating cost-effective method of olfactory training in clinical practice: Essential oils at uncontrolled concentration. Laryngoscope Investigative Otolaryngology, 2(2), 53–56. https://doi.org/10.1002/lio2.62
Pause, B. M., Raack, N., Sojka, B., Göder, R., Aldenhoff, J. B., & Ferstl, R. (2003). Convergent and divergent effects of odors and emotions in depression. Psychophysiology, 40(2), 209–225. https://doi.org/10.1111/1469-8986.00023
Pellegrini, M., Bernabei, F., Schiavi, C., & Giannaccare, G. (2020). Impact of cataract surgery on depression and cognitive function: Systematic review and meta-analysis. Clinical & experimental ophthalmology, 48(5), 593–601. https://doi.org/10.1111/ceo.13754
Pellegrino, R., Han, P., Reither, N., & Hummel, T. (2019). Effectiveness of olfactory training on different severities of posttraumatic loss of smell. The Laryngoscope, 129(8), 1737–1743. https://doi.org/10.1002/lary.27832
Pinto, J. M., Wroblewski, K. E., Kern, D. W., Schumm, L. P., & McClintock, M. K. (2014). Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE, 9(10), e107541. https://doi.org/10.1371/journal.pone.0107541
Pinto, J. M., Wroblewski, K. E., Huisingh-Scheetz, M., Correia, C., Lopez, K. J., Chen, R. C., Kern, D. W., Schumm, P. L., Dale, W., & McClintock, M. K. (2017). Global sensory impairment predicts morbidity and mortality in older U.S. adults. Journal of the American Geriatrics Society, 65(12), 2587–2595. https://doi.org/10.1111/jgs.15031
Poletti, S. C., Michel, E., & Hummel, T. (2017). Olfactory training using heavy and light weight molecule odors. Perception, 46(3–4), 343–351. https://doi.org/10.1177/0301006616672881
Porada, D. K., Regenbogen, C., Seubert, J., Freiherr, J., & Lundström, J. N. (2019). Multisensory enhancement of odor object processing in primary olfactory cortex. Neuroscience, 418, 254–265. https://doi.org/10.1016/j.neuroscience.2019.08.040
Rai, N., Hipolito, M. M., VanMeter, J. W., Seth, R., Adenuga, A., Shelby, M., Misiak-Christian, M., Nwaokobia, C., Manaye, K. F., Obisesan, T. O., & Nwulia, E. (2021). Comparative effects of repetitive odor identification and odor memory tasks on olfactory engagement in older populations - A pilot fMRI study. Neuropsychiatric Disease and Treatment, 17, 1279–1288. https://doi.org/10.2147/NDT.S298303
Reichert, J. L., & Schöpf, V. (2018). Olfactory loss and regain: Lessons for neuroplasticity. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 24(1), 22–35. https://doi.org/10.1177/1073858417703910
Rezaeyan, A., Asadi, S., Kamrava, S. K., Khoei, S., & Zare-Sadeghi, A. (2022). Reorganizing brain structure through olfactory training in post-traumatic smell impairment: An MRI study. Journal of Neuroradiology, 49(4), 333–342. https://doi.org/10.1016/j.neurad.2021.04.035
Roalf, D. R., Moberg, M. J., Turetsky, B. I., Brennan, L., Kabadi, S., Wolk, D. A., & Moberg, P. J. (2017). A quantitative meta-analysis of olfactory dysfunction in mild cognitive impairment. Journal of Neurology, Neurosurgery, and Psychiatry, 88(3), 226–232. https://doi.org/10.1136/jnnp-2016-314638
Ross, L. A., Edwards, J. D., O'Connor, M. L., Ball, K. K., Wadley, V. G., & Vance, D. E. (2016). The Transfer of Cognitive Speed of Processing Training to Older Adults' Driving Mobility Across 5 Years. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 71(1), 87–97. https://doi.org/10.1093/geronb/gbv022
Ross, L. A., Freed, S. A., Edwards, J. D., Phillips, C. B., & Ball, K. (2017). The Impact of Three Cognitive Training Programs on Driving Cessation Across 10 Years: A Randomized Controlled Trial. The Gerontologist, 57(5), 838–846. https://doi.org/10.1093/geront/gnw143
Ross, L. A., Saint-Amour, D., Leavitt, V. M., Javitt, D. C., & Foxe, J. J. (2007). Do you see what I am saying? Exploring visual enhancement of speech comprehension in noisy environments. Cerebral Cortex, 17(5), 1147–1153. https://doi.org/10.1093/cercor/bhl024
Rotton, J. (1983). Affective and cognitive consequences of malodorous pollution. Basic and Applied Social Psychology, 4, 171–191.
Rottstaedt, F., Weidner, K., Hummel, T., & Croy, I. (2018a). Pre-aging of the OB in major depression with high comorbidity of mental disorders. Frontiers in Aging Neuroscience, 10, 354. https://doi.org/10.3389/fnagi.2018.00354
Rottstaedt, F., Weidner, K., Strauss, T., Schellong, J., Kitzler, H., Wolff-Stephan, S., Hummel, T., & Croy, I. (2018b). Size matters - The OB as a marker for depression. Journal of Affective Disorders, 229, 193–198. https://doi.org/10.1016/j.jad.2017.12.047
Salehpour, F., Hamblin, M. R., & DiDuro, J. O. (2019). Rapid reversal of cognitive decline, olfactory dysfunction, and quality of life using multi-modality photobiomodulation therapy: Case report. Photobiomodulation, Photomedicine, and Laser Surgery, 37(3), 159–167. https://doi.org/10.1089/photob.2018.4569
Savic, I. (2002). Imaging of brain activation by odorants in humans. Current Opinion in Neurobiology, 12(4), 455–461. https://doi.org/10.1016/s0959-4388(02)00346-x
Schmahmann, J. D. (2019). The cerebellum and cognition. Neuroscience Letters, 688, 62–75. https://doi.org/10.1016/j.neulet.2018.07.005
Schubert, C. R., Carmichael, L. L., Murphy, C., Klein, B. E., Klein, R., & Cruickshanks, K. J. (2008). Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. Journal of the American Geriatrics Society, 56(8), 1517–1521. https://doi.org/10.1111/j.1532-5415.2008.01826.x
Segura, B., Baggio, H. C., Solana, E., Palacios, E. M., Vendrell, P., Bargalló, N., & Junqué, C. (2013). Neuroanatomical correlates of olfactory loss in normal aged subjects. Behavioural Brain Research, 246, 148–153. https://doi.org/10.1016/j.bbr.2013.02.025
Sinding, C., Puschmann, L., & Hummel, T. (2014). Is the age-related loss in olfactory sensitivity similar for light and heavy molecules. Chemical Senses, 39, 383–390. https://doi.org/10.1093/chemse/bju004
Sorokowski, P., Karwowski, M., Misiak, M., Marczak, M. K., Dziekan, M., Hummel, T., & Sorokowska, A. (2019). Sex differences in human olfaction: A meta-analysis. Frontiers in Psychology, 10, 242. https://doi.org/10.3389/fpsyg.2019.00242
Takahashi, T., Nishikawa, Y., Yücel, M., Whittle, S., Lorenzetti, V., Walterfang, M., Sasabayashi, D., Suzuki, M., Pantelis, C., & Allen, N. B. (2016). Olfactory sulcus morphology in patients with current and past major depression. Psychiatry Research. Neuroimaging, 255, 60–65. https://doi.org/10.1016/j.pscychresns.2016.07.008
Tsushima, Y., Nishino, Y., & Ando, H. (2021). Olfactory stimulation modulates visual perception without training. Frontiers in Neuroscience, 15, 642584. https://doi.org/10.3389/fnins.2021.642584
Uchida, Y., Sugiura, S., Nishita, Y., Saji, N., Sone, M., & Ueda, H. (2019). Age-related hearing loss and cognitive decline – The potential mechanisms linking the two. Auris, Nasus, Larynx, 46, 1–9. https://doi.org/10.1016/j.ani.2018.08.010
Uddin, L. Q., Nomi, J. S., Hébert-Seropian, B., Ghaziri, J., & Boucher, O. (2017). Structure and function of the human insula. Journal of Clinical Neurophysiology, 34(4), 300–306. https://doi.org/10.1097/WNP.0000000000000377
Vance, D. E., & Brew, B. J. (2021). HIV neurocognitive impairment and Alzheimer’ disease: Sniffing out the difference. AIDS, 35(3), 511–513. https://doi.org/10.1097/QAD.0000000000002731
Vance, D., Kaur, J., Fazeli, P. L., Talley, M. H., Yuen, H. K., Kitchin, B., & Lin, F. (2012). Neuroplasticity and successful cognitive aging: A brief overview for nursing. Journal of Neuroscience Nursing, 44(4), 218–227. https://doi.org/10.1097/JNN.0b013e3182527571
Vance, D. E., Lee, L., Munoz-Moreno, J. A., Morrison, S., Overton, T., Willig, A., & Fazeli, P. L. (2019). Cognitive reserve over the lifespan: Neurocognitive implications for aging with HIV. Journal of the Association of Nurses in AIDS Care, 30(5), e109–e121. https://doi.org/10.1097/JNC.0000000000000071
Vance, D. E., Nicholson, C., Cheatwood, J., Morrison, S., & Fazeli, P. L. (2020). Olfactory dysfunction in aging African American and Caucasian men with HIV: A pilot study. Journal of the Association of Nurses in AIDS Care. https://doi.org/10.1097/JNC.0000000000000061
Vance, D. E., Perazzo, J. D., & Fazeli, P. L. (2021). Parallels between neuroHIV and neuroCOVID-19: Considerations for a post-COVID-19 era. Journal of the Association of Nurses in AIDS Care, 32(5), e55–e59. https://doi.org/10.1097/JNC.0000000000000265
Vanderwolf, C. H. (1992). Hippocampal activity, olfaction, and sniffing: An olfactory input to the dentate gyrus. Brain Research, 593(2), 197–208. https://doi.org/10.1016/0006-8993(92)91308-2
Varadaraj, V., Munoz, B., Deal, J. A., An, Y., Albert, M. S., Resnick, S. M., Ferrucci, L., & Swenor, B. K. (2021). Association of Vision Impairment With Cognitive Decline Across Multiple Domains in Older Adults. JAMA network open, 4(7), e2117416. https://doi.org/10.1001/jamanetworkopen.2021.17416
Wabnegger, A., & Schienle, A. (2019). Cerebellar gray matter and olfactory performance. Chemical Senses, 44(7), 507–510. https://doi.org/10.1093/chemse/bjz045
Woodward, M. R., Amrutkar, C. V., Shah, H. C., Benedict, R. H., Rajakrishnan, S., Doody, R. S., Yan, L., & Szigeti, K. (2017). Validation of olfactory deficit as a biomarker of Alzheimer disease. Neurology: Clinical Practice, 7(1), 5–14. https://doi.org/10.1212/CPJ.0000000000000293
Woodward, M. R., Hafeez, M. U., Qi, Q., Riaz, A., Benedict, R., Yan, L., Szigeti, K., Texas Alzheimer’s Research, Care Consortium. (2018). Odorant item specific olfactory identification deficit may differentiate Alzheimer disease from aging. The American Journal of Geriatric Psychiatry, 26(8), 835–846. https://doi.org/10.1016/j.jagp.2018.02.008
Yaffe, K., Freimer, D., Chen, H., Asao, K., Rosso, A., Rubin, S., Tranah, G., Cummings, S., & Simonsick, E. (2017). Olfaction and risk of dementia in a biracial cohort of older adults. Neurology, 88(5), 456–462. https://doi.org/10.1212/WNL.0000000000003558
Yao, L., Pinto, J. M., Yi, X., Li, L., Peng, P., & Wei, Y. (2014). Gray matter volume reduction of olfactory cortices in patients with idiopathic olfactory loss. Chemical Senses, 39(9), 755–760. https://doi.org/10.1093/chemse/bju047
Funding
Dr. Vance and Fazeli are supported by an NIH/National Institute of Mental Health R01-award (1R01MH106366-01A1). Dr. Kamath is supported by grants from the National Institute of Health (R01AG064093 and R01NS108452). Dr. Dy Cho is supported by grants from NIH/National Institutes of Allergy and Infectious Disease (NIAID; K084AI46220).
Author information
Authors and Affiliations
Contributions
All authors listed met the ICMJE authorship criteria: (a) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting the manuscript or revising it critically for important intellectual content; (c) final approval of the version to be published; and (d) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Specifically, all authors were involved with the following: 1) Conceptualization; 2) Writing (original draft), and 3) Writing (review and editing).
Corresponding author
Ethics declarations
Ethical Approval
This is a systematic review of published articles in the public domain. Ethical or human subjects approval is not required.
Competing Interests
The authors report no real or perceived vested interest that relate to this article that could be construed as a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1 Narrative Summaries of Olfactory Training Studies that Examined Cognition or Brain Function
Appendix 1 Narrative Summaries of Olfactory Training Studies that Examined Cognition or Brain Function
Cognitive Studies
-
1.
Birte-Antina et al. (2018) – Cognition Study
In a two-group pre-post experimental design study, Birte-Antina et al. (2018) examined the effects of OT on improved olfactory function, cognitive function, and overall well-being in older people. Ninety-one older adults (Mage = 61.1 yrs) with normal olfaction and no neurodegenerative or metabolic diseases were randomized into two groups: an OT group (n = 60) or a Sudoku control group (n = 31). Odor detection threshold, odor discrimination, and odor identification were tested pre- and post-training with the Sniffin’ Sticks test. They were also administered cognitive performance measures, including the Montreal Cognitive Assessment (MoCA), the Controlled Oral Word Association Test (COWAT), the Auditory Verbal Learning Test, and the d2 attention and concentration test. For five months, the OT group completed training 2x/day which consisted of smelling the odorants of lime, cloves, eucalyptus, and rose. Participants recorded the intensity with which they perceived each odorant in diaries which served as a training log. The control group completed Sudoku puzzles 2x/day from a book that served as their training log.
Results indicated that TDI scores for the OT group improved following training (t = 6.8, p < 0.001), with 20% showing clinically relevant improvement, while TDI scores for the control group were unchanged. Specifically, the OT group improved in odor detection threshold and odor discrimination but not in odor identification. Cognitively, the OT group showed statistically significant improvement in the semantic (category-guided) verbal fluency subtest of the COWAT (t = 5.8, p < 0.001) and greater improvement than the control group on the short-term memory portion of the MoCA test (t = -6.7, p < 0.001). The OT group also experienced a decrease in depressive symptomatology (z = -2.21, p = 0.02) and an increase in wellbeing (i.e., WHO Well-Being Index) (t = 2.5, p = 0.02) compared to the control group. Unfortunately, this study was limited by unknown adherence to the OT despite the use of daily diaries. Like other studies, this study lacked longitudinal follow-up after training. The strengths of this study included the use of an active control group and sufficient sample size with demonstrated OT effectiveness in a sample of older adults without olfactory loss.
-
2.
Oleszkiewicz et al. (2021) – Cognition Study
In a three-group pre-post experimental design study, Oleszkiewicz et al. (2021) examined: 1) the effects of OT on cognitive and emotional variables, and 2) whether single molecule odorants or more complex multi-molecule odorant mixtures would create more of a therapeutic effect. Sixty-eight healthy older adults (Mage = 62.8 yrs; 50 – 84 yrs) “at-risk” of age-related olfactory decline were randomized into three groups: 1) “simple” single molecule OT group (n = 26), 2) odorant “mixture” OT group (n = 27), or 3) a no-contact control group (n = 15). Those in the “simple” OT group received 9 single-molecule odorants (e.g., eugenol, menthol) to smell and those in the “mixture” OT group received 9 odorant mixtures (e.g., peppermint oil, clove bud essential oil); both OT groups were instructed to sniff each odorant 2x/day for 20 s; training duration ranged from 3 to 6 months (Mmonths = 4.13). All participants completed baseline olfactory function testing using Sniffin’ Sticks test to receive a TDI score and underwent medical evaluation for medical issues that could lead to diminished olfactory function. Cognitive testing included the MoCA, Dementia Screening Interview (AD8), COWAT; emotional testing was also included (i.e., Beck Depression Inventory & Positive and Negative Affect Schedule).
Following OT, analysis of variance revealed that the “simple” OT group showed an improved olfactory threshold, but the mixtures and control groups did not (F(2,56) = 3.31, p = 0.044). There were no effects of OT on odor discrimination or identification. AD8 dementia screening scores worsened in the control group while no decline was seen in either OT groups (F(2, 65) = 4, p = 0.029). Lastly, MoCA testing scores were found to be improved in the “simple” OT group after training while the control group and the “mixture” OT group experienced no changes (F(2,54) = 3.18, p = 0.49). In summary, improvements on cognitive scores were observed after training in simple odorants, but using more complex odorants in OT did not result in greater olfactory or cognitive benefit.
-
3.
Oleszkiewicz et al. (2022) – Cognition Study
In a two-group pre-post experimental design, Oleszkiewicz et al. (2022) examined the effect of OT dose, sniffing odorants 2x/day versus 4x/day, on both cognitive and olfactory outcomes. Participants were 26 hyposmic/anosmic adults with either infectious or post-traumatic etiology (Mage = 59.2 yrs) and a control group of 29 normosmic adults (Mage = 57.4 yrs). The hyposmic/anosmic participants underwent a medical screening process to exclude comorbidities that could interfere with OT (i.e., diabetes, recent infection, or chronic sinonasal problems). Baseline olfactory function was established with the Sniffin’ Sticks test (TDI score of > 30.5 was classified as a normosmic). All participants completed the following cognitive tests: MoCA, COWAT, and a timed semantic verbal fluency task All participants were randomized to receive either the standard (sniffing odorant 2x/day) OT or the intense (4x/day) OT. The OT involved using an odorant dispenser that released 4 mL of the odorized air with every push of a button. Participants were instructed to sniff for 30 s with repeat button pushes as needed. For the first three months, initial odorants were grapefruit, lavender, peppermint, lemon grass, and ylang-ylang (a tropical flower). Three months later, odorants were changed to green tea, bergamot (a citrus fruit), menthol, thyme, and tangerine. The training took place every 6 (4x/day group) or 12 (2x/day group) hours depending on group assignment. Unlike other OT studies, participants were instructed not to engage in OT 30 min before or after meals, apparently to not confound delivery of OT with the smell of foods.
Analysis revealed that the standard OT group showed significant improvement in odor detection threshold that was greater than the improvement experienced by the intense OT group. Interestingly, participants with and without olfactory impairment benefitted significantly from OT. In addition, there was a significant interaction between dose and improvement in semantic fluency (F = 18.40, df = 1.48, p < 0.001) such that the group receiving standard OT showed greater improvement than the group receiving intense OT. Moreover, a longer duration of OT was correlated with smaller increases in improved olfactory detection threshold. The verbal semantic fluency task showed similar effects in the standard OT group with significant improvement post-OT, while the intense olfactory group did not show improvement. Similarly, MoCA scores were negatively correlated with shorter OT duration (r = -0.36, p = 0.01) and increased olfactory sensitivity (r = 0.33, p = 0.02). In summary, OT performed at a standard dose of 2x/day was more effective than OT at a more intense dose of 4x/day at improving both olfactory function (threshold) and cognition (semantic verbal fluency). Several study limitations may explain this result, including unequal distribution of olfactory disorder etiologies and differences in baseline olfactory abilities across groups. Indeed, the standard group had worse performance in odor detection thresholds at baseline compared to the intense group. This study was also limited by the small sample sizes, unreported adherence, and lack of an imaging component. Strengths included long duration of OT, variation of doses, the inclusion of OT for the normosmic control group, and the introduction of new odorants midway through the study.
-
4.
Cha et al. (2022) – Cognition Study
In a two-group pre-post experimental design, Cha et al. (2022) examined the effects of a short-term intense OT program on cognition in dementia patients in Korea. The study included adults with dementia with 34 participants in the intensive OT regimen (Mage = 85 yrs, range: 65–97) and 31 controls (Mage = 85 yrs, range: 65–100). All participants completed baseline testing of olfactory function using YSK Olfactory Function (YOF) test to measure odor identification, nine cognitive tests, and other measures. The intensive OT consisted of 40 essential oils used 2x/day for 15 days. For each odor, two drops of oil were added to a sponge in a flexible 20-mL container which was squeezed 2–3 cm away from the participants’ nose and held in place for 5 s; this was repeated for all forty odors.
Following training, the intensive OT group demonstrated significant improvements in verbal fluency test (p = 0.001), Korean version of Boston Naming Test (p = 0.001), Word List Memory Test (p < 0.001), Word List Recall test (p = 0.031), and Word List Recognition Test (p < 0.001). Overall, the study showed increases in the cognitive domains of attention, memory, and language, and demonstrated significant improvement in depression (i.e., SGDS-K). This study has several limitations including a short training period, a small sample size, lack of long-term follow-up, and lack of differentiation among types of dementia.
Neuroimaging Studies
-
5.
Kollndorfer et al. (2014) – Neuroimaging Study (Functional Connectivity)
In a one-group pre-post experimental design study conducted over three months, Kollndorfer et al. (2014) examined the effects of OT on olfaction and neural connectivity as measured by fMRI in anosmia participants with complete olfactory loss caused by upper respiratory tract infections (URTI). More specifically, the study explored the changes in functional connectivity in major olfactory areas, the right and left pyriform cortices (see Fig. 2), using fMRI during a sniffing paradigm following OT. Study recruitment, and exclusions due to incomplete MRI data, led to the inclusion of seven participants (n = 7; Mage = 41.6 yrs, mean disease duration = 4.6 yrs). Olfactory loss was confirmed by an otolaryngologist, who assessed olfactory function using the Sniffin’ Sticks test battery. For the OT, participants selected four out of six odorants (cinnamon, vanilla, orange, rose, menthol, banana) to include in their training; they were instructed to “take one deep sniff of every odor” from a brown glass jar 2x/day for 12 weeks. Compliance was verified through weekly follow-up calls and daily diaries; all participants reported completing the training protocol as directed.
Results indicated significant improvement in odor detection threshold (p = 0.028), though improvement in odor discrimination and odor identification was not significant. fMRI data showed altered functional connectivity of the pyriform cortices following OT. At baseline, widespread network engagement was needed to process olfaction; this network included areas such as the left inferior frontal gyrus and the left premotor cortex which are outside the olfactory regions. Following OT, only one significant connection with the piriform cortex was retained, and that was to the right subgenual cortex; meanwhile, engagement of the pyriform cortices with non-olfactory functional connections declined, leaving only one significant connection to the right subgenual cortex. This study demonstrated that high plasticity in the pyriform cortex may be a fundamental component to improved odor detection threshold observed with OT. Further, the impact of olfactory loss exceeds just the olfactory processing areas within the brain. Regarding the lack of changes in discrimination and identification, prior research suggests that these may require higher levels of cognition, and thus may improve after more extended periods of OT. Limitations of this study include relatively short duration of the training, lack of cognitive measures, and a small sample size limiting generalizability.
-
6.
Kollndorfer et al. (2015) – Neuroimaging Study (Functional Connectivity)
Kollndorfer et al. (2015) aimed to clarify the underlying mechanisms of brain reorganization observed in their prior study. In the first of two studies, healthy adult normosmic controls and anosmia patients were compared on fMRI during delivery of three different odorants (i.e., CO2, cinnamaldehyde, & menthol) to the left nostril (in 500 ms bursts with an inter-stimulus interval of 30 s for a total scanning time of 30 min). Results clarified that there was no difference in observed brain activity among the odorants. Despite the lack of conscious odor perception, the same overlapping neural networks (olfactory, somatosensory, & integrative based on trigeminal processing) were activated in response to the odorant presentation. Curiously, symmetrical brain activation of the olfactory network was observed despite odorant delivery to the left nostril. Importantly, participants with anosmia had significantly less functional connectivity during odorant delivery than the normosmic control group within all three neural networks. This suggests that reduced olfactory stimulation experienced by the participants with anosmia resulted in less neuroplasticity during training, and conversely, that high plasticity of the olfactory system may explain the success of OT reported by Kollndorfer et al. (2014).
More relevant to the focus of this systematic review, in the one-group pre-post experimental design, the same anosmic participants as in Kollndorfer et al. (2014) completed an OT intervention consisting of exposure to four odorants (selected from a choice of six odorants) impregnated on cotton balls for 12 weeks 2x/day with instructions to take one deep sniff of each odorant. Olfaction was assessed by the Sniffin’ Sticks test. fMRI was acquired at baseline and posttest OT. During fMRI, stimuli were applied to the left nostril via a single air-line with a nose applicator.
As in Kollndorfer et al. (2014), odor detection threshold, but not odor fMRI identification or odor discrimination, was significantly improved following OT. Furthermore, after OT, a change in activity within the brain was observed by 3.1% in the olfactory network, 1.6% in the somatosensory network, and 4.2% in the integrative network. After OT, an increase in functional connectivity was observed for all three networks: for the olfactory network (from 0 to 4 connections), somatosensory network (from 8 to 10 connections, increased 25%), and the integrative network (from 13 to 22 connections, increased 69%).
In summary, this study demonstrated that three odorants were processed by similar neuronal networks. In addition, fMRI revealed that the same networks were activated in anosmic participants as in healthy controls despite the inability of the anosmic participants to consciously perceive the odorants. Limitations of this study include the lack of any subjective or objective cognitive measures and the small sample size which limits generalizability.
-
7.
Negoias et al. (2017) – Neuroimaging Study (Olfactory Bulb)
In a one-group pre-post experimental design, Negoias et al. (2017) examined olfactory bulb volumetric MRI changes before and after lateralized OT in normosmic participants. The study included 97 participants (Mage = 23.74 yrs) with no history of olfactory impairment or conditions that could interfere with olfactory function, as confirmed by nasal endoscopy. Over a 4-month training period, one-nostril OT (randomized to left or right side) was conducted by exposing the same nostril 2x/day for ten seconds to each of four odorants (lemon, rose, eucalyptus, and cloves) while closing the other nostril. Participant interest and adherence were encouraged by using a training diary. Olfactory bulb volume before and after OT was measured with MRI. Participants were administered lateralization odor detection threshold and odor identification testing, but not odor discrimination, using the Sniffin’ Sticks test. Contrary to expectation, a significant decline in odor detection threshold scores was observed for both the trained nostril (p < 0.001) and the untrained nostril (p < 0.005). There was no effect of training on olfactory identification scores. Despite the decline in odor detection threshold, olfactory bulb volume increased after OT for both the trained (11.3%) and untrained (13.1%) nostrils, suggesting that central top-down mechanisms are most likely involved.
In summary, a longitudinal measure of olfactory bulb volume before and after OT is an innovative aspect of this study. The primary finding is the neuroplasticity in the olfactory bulb demonstrated by MRI following OT in normosmic young adults. The increase in olfactory bulb volume in both the trained and untrained nostrils is especially interesting because it suggests that more than direct sensory stimulation of the bulb is involved in plasticity, implying top-down processes are at work in OT. The absence of improved performance on Sniffin’ Sticks odor detection or identification subscales may be due to overexposure to stimuli, adaptation, loss of interest and engagement in the training (despite documented adherence), or to a possible ceiling effect in individuals who perform in the above-average range during baseline odor threshold assessment.
-
8.
Gellrich et al. (2018) – Neuroimaging Study (Olfactory Bulb)
In a two-group pre-post experimental design study over three months, Gellrich et al. (2018) examined the effects of OT on improved olfactory function and brain structure via structural MRI and fMRI in a URTI-related hyposmia group (n = 31; Mage = 53.5 yrs) and a non-hyposmic control group (n = 30; Mage = 60.7 yrs). The control group was not administered the OT or the posttest assessment. Sniffin’ Sticks tests were used to assess olfactory function. The odor detection threshold was measured through a blindfolded trial of a three-alternative forced choice procedure using diluted phenylethyl alcohol. The OT group underwent 12 weeks of training 2x/day, sniffing four specified odorants (rose, eucalyptus, lemon, cloves) individually for 10 s with participants instructed to focus on the odorant.
After OT, the hyposmia group had significantly improved scores for odor detection threshold (p = 0.004), odor discrimination (p = 0.005), odor identification (p = 0.007), and overall TDI (p < 0.001). Fifty-three percent of the hyposmia group showed clinically relevant improvement. After OT, the hyposmia group also showed increased grey matter volume in the thalamus, cerebellum, and hippocampus. A trend was observed for an olfactory bulb volume increase after OT. In summary, this study highlighted the finding that OT promotes neuroplastic changes. Study limitations included a small sample size, unknown adherence, lack of cognitive assessment, and absence of an adequate control group.
-
9.
Al Aïn et al. (2019) – Neuroimaging Study (Structural)
Using an extremely well-controlled three-group pre-post experimental design, Al Aïn et al. (2019) examined the effects of OT on task specific and intramodal olfactory performance and MRI measures of cortical thickness. The sample consisted of 36 normosmic participants (Mage = 24 yrs) who were randomly divided into three groups consisting of OT (n = 12), visual training (n = 12), and no training (n = 12). In this study, unlike the other studies reported in this review, OT consisted of 20–30 min sessions conducted in the laboratory for six weeks. Training involved an odor intensity classification task, an odor quality classification task, and a target odor detection task. Odor intensity classification consisted of ordering 16 odor samples of the target odorant from lightest to strongest. Odor quality classification consisted of ordering 11 odor samples according to samples of the target odor mixed with citrus. Target odor detection consisted of identifying whether the target odor was present in each of a sample of 14 bottles. Visual training consisted of three analogous tasks involving colored paper and was conducted to ensure that any olfactory improvement was due to OT and not the training process itself. Both the OT and visual training groups underwent olfactory assessment of odor threshold (for PEA and n-butanol odorants), odor discrimination, odor identification (free and cued), and odor memory before and following OT. Both the OT and visual training groups also received structural MRI before and following training.
Analysis revealed no difference in olfactory performance between the visual training nd non-training control groups which were then combined. Following training, the OT group showed a significant benefit of training on the trained tasks and, in addition, performed significantly better than the control groups on the six non-trained tasks and free odor identification. Analysis of MRI data revealed that the OT group showed a significant increase in cortical thickness in the right inferior frontal gyrus, the bilateral fusiform gyrus, and the right entorhinal cortex. Finally, there was a weak positive association between increased occipital thickness and olfactory memory. In summary, this study is unique in using a highly controlled method for OT and two control groups, as well as a different program of training than other studies. OT resulted in improved olfactory performance on the trained tasks that generalized to other olfactory tasks and increased regions of olfactory thickness. Study limitations include the small sample size and relatively short duration of training.
-
10.
Pellegrino et al. (2019) – Neuroimaging Study (Functional Connectivity)
In a one-group pre-post experimental design over six months, Pellegrino et al. (2019) examined the effects of OT in 37 (originally 42) participants with traumatic brain injury (Mage = 52.2 year; range: 23–74 yrs) through objective psychophysical olfactory assessment, olfactory bulb volume via structural MRI, and connectivity via fMRI. The sample was divided based on the degree of olfactory loss using the Sniffin’ Sticks test battery, resulting in 14 hyposmic and 23 anosmic participants. Both groups underwent the same OT for a minimum of 24 weeks (Mmonths = 7.1) which consisted of sniffing cotton balls impregnated with four odorants (rose, eucalyptus, lemon, and cloves) for approximately 15 s 2x/day. During fMRI acquisition, participants were exposed to either a peach or a coffee odorant and intermittently asked to identify the odorant, its intensity, and pleasantness. After six months, follow-up structural MRI and fMRI were completed using the same method.
After OT, overall TDI (t[36] = 2.85, p = 0.007) scores and odor identification (t[36] = 2.14, p = 0.04) and odor threshold (t[36] = 2.33, p = 0.03) scores increased for the two groups combined, but there was no change in odor discrimination scores. Anosmic participants showed a significant increase in TDI following training while hyposmic patients showed only a trend. The structural MRI component of the study did not show considerable olfactory bulb volume changes post- training for either the anosmic or hyposmic participants. Yet, fMRI revealed that hyposmic participants showed more areas of brain activity in response to odorants than before training, including the dorsal anterior cingulate cortex and several left hemisphere structures. In addition, the anosmic group experienced significantly increased activation of the right superior frontal gyrus after training. This study is unusual in reporting adherence to the OT protocol (i.e., 50% reported OT adherence as instructed, 40% reported OT 1–2 times/day, and 10% reported OT less than 7x/week); however, adherence was not related to study outcomes. Study limitations include the lack of a normosmic control group, lack of no training control group, and a small number of participants. In summary, TDI improvement and increased brain activation were observed in the absence of an increase in olfactory bulb volume. This pattern is not compatible with a purely “bottom-up” mechanism of OT but rather suggests that OT may be triggering a top-down component of neuroplasticity.
-
11.
Hosseini et al. (2020) – Neuroimaging Study (Functional Connectivity)
In a two-group pre-post experimental design over 16 weeks, Hosseini et al. (2020) examined the effects of OT on resting-state connectivity among neural regions known to be involved in olfactory processing. The areas of focus were the amygdala, piriform cortex, insula, cingulate cortex, and orbital frontal cortex. Participants were 16 adults with smell dysfunction (14 anosmic, two hyposmic) resulting from traumatic brain injury. Olfactory performance was measured at baseline and following OT using the Sniffin’ Sticks test. OT consisted of sniffing four odorants (rose, eucalyptus, lemon, thyme) 2x/day for five minutes (rotating odorants every ten seconds) for 16 weeks.
There were no group differences in smell performance at baseline. Following OT, the treatment group improved performance on odor discrimination and overall TDI but not on odor detection threshold or odor identification. Resting state fMRI revealed changes in connectivity among the five neural regions of interest following OT. Compared to the control group, the OT group showed stronger connections from the cingulate cortex to the insula, increased connectivity within the orbital frontal cortex, and diminished connectivity from the orbital frontal cortex to the cingulate cortex. Study limitations include small sample size, lack of reported adherence, and lack of a normosmic control group.
-
12.
Mahmut et al. (2020) – Neuroimaging Study (Olfactory Bulb)
Mahmut et al. (2020) reported on two studies. In the cross-sectional study 1, healthy controls (n = 27; Mage = 65.3 yrs) and adults with idiopathic olfactory loss (n = 27; Mage = 66.1 yrs) were compared on structural MRI and olfaction. As expected, healthy controls had a significantly larger right (F(1,53) = 25.02, p < 0.0001) and left ([F(1,53) = 12.53, p < 0.001) olfactory bulb volumes than those with idiopathic olfactory loss; likewise, healthy controls also had better odor detection threshold (p < 0.0001), odor discrimination (p < 0.0001), odor identification (p < 0.0001), and overall TDI (p < 0.0001) than those with idiopathic olfactory loss.
In the one-group pre-post experimental design of study 2, the same sample of adults with idiopathic olfactory loss (n = 27; Mage = 66.1 yrs) engaged in OT which involved smelling each of the four odorants (rose, eucalyptus, lemon, cloves) 2x/day for approximately six months (Mdays = 213). Following OT, structural MRI revealed olfactory bulb volume growth bilaterally, with both right olfactory bulb volume (t(26) = 3.04, p = 0.005), and left olfactory bulb volume showing significant change from baseline (t(26) = 2.11, p = 0.045). In addition to olfactory bulb volume increase, 22% of participants had clinically meaningful improvement in olfactory function. Odor identification (t(26) = 3.47, p = 0.002) and overall TDI (t(26) = 3.45, p = 0.002) significantly improved following OT; however, odor discrimination and odor threshold were not improved. In summary, this study provided a long period of OT, and the results supported expectations that OT does improve olfaction, which is reflected in neuroplastic growth of olfactory bulb volume. Study limitations include lack of a control arm for the second study, a small number of participants, unknown adherence, and absence of a cognitive component.
-
13.
Han et al. (2021) – Neuroimaging Study (Structural)
In a two-group pre-post experimental design over seven months, Han et al. (2021) examined the effects of OT on olfactory performance and structural MRI in 24 patients (12 anosmic and 12 hyposmic) with idiopathic olfactory loss; 30 normosmic adults were included at baseline as controls. Olfactory performance was measured at baseline and following OT using the Sniffin’ Sticks test. OT consisted of sniffing four odorants (rose, lemon, eucalyptus, cloves) 2x/day for 20–30 s each for seven months. The normosmic control group did not receive OT. All participants underwent MRI scanning at baseline and follow-up.
Analysis of baseline olfactory performance revealed a significant group effect for odor identification, odor discrimination, and odor detection such that healthy controls performed better than the idiopathic olfactory loss group. The idiopathic olfactory loss group showed significant improvement in odor identification following OT but not in TDI or odor detection or odor discrimination. Analysis of MRI data for baseline differences in grey matter volume revealed that the idiopathic olfactory loss group showed larger grey matter volume in the lateral orbitofrontal cortex. There was no brain region in which the healthy control group showed larger grey matter volume.
At follow-up MRI in the idiopathic olfactory loss group following OT, voxel-based morphometry revealed increased grey matter volume in several regions including the cerebellum (bilateral), thalamus (bilateral), right superior frontal cortex, right supplementary motor area, right medial orbital frontal cortex, and the right gyrus rectus. In addition, idiopathic olfactory loss participants with anosmia significantly showed increased grey matter volume in additional brain regions including the left precuneus, left superior frontal medial cortex, and the left midcingulate cortex following OT, regions previously suggested to be involved with olfactory memory. Yet, no significant correlation emerged between increased grey matter volume and improved odor identification scores in the idiopathic olfactory loss group. Study limitations include absence of adherence data and lack of appropriate control groups.
-
14.
Jiramongkolchai et al. (2021) – Neuroimaging Study (Functional Connectivity)
In a one group pre-post experimental design, Jiramongkolchai et al. (2021) examined the effect of OT in participants with postviral olfactory dysfunction (PVOD) by using fMRI to measure neural connectivity. Sixteen adults (Mage = 60 yrs, range: 30–70) with PVOD of three months or longer were paired with matched controls (n = 20) at baseline. Initial olfactory testing consisted of the University of Pennsylvania Smell Identification Test (UPSIT) and Sniffin’ Sticks. Eligible participants underwent one-month-long budesonide nasal irrigation to decrease any inflammation that could be contributing to PVOD. Baseline fMRI charted a total of 187 regions of interest (ROI), with a follow up scheduled after completion of OT. Participants then underwent 12 weeks of OT consisting of 4 oils (rose, eucalyptus, lemon, and clove) in 1 mL vials that were used 2x/day for 20–30 s each time over a 3-month period with a corresponding diary to track progress. The study concluded with a post OT test and fMRI in the PVOD participants only.
At baseline fMRI, there were 13 ROI that differed significantly between PVOD group and controls primarily in the visual cortex, cerebellum, and angular gyrus. After OT, 5 network connections demonstrated changes, with increased connections between inferior temporal gyrus with inferior frontal gyrus (p = 0.02), occipital junction with cerebellum (p = 0.01), and decreased connections between inferior temporal gyrus with the medial visual network (p = 0.04), the lingual gyrus (p = 0.03), and the occipital pole (p = 0.04). Following OT, nine of the sixteen participants had clinically significant improvement in smell with median change in UPSIT of 1.5 and median change in TDI of 1.25. Overall, the study demonstrated that patients with PVOD had several differences of brain connectivity with the control group at baseline, and the PVOD group created additional connections in olfactory areas of the brain after OT. Study limitations included a small sample size, lack of long-term follow-up, and no experimental control group.
-
15.
Gürbüz et al. (2022) – Neuroimaging Study (Olfactory Bulb)
In a unique within group pre-post design study, Gürbüz et al. (2022) examined the effect of OT training on olfactory performance and olfactory bulb volume in patients at least five years following total laryngectomy for cancer. Patients with total laryngectomy often experience olfactory dysfunction, possibly due to a lack of airflow to olfactory regions. These patients have been noted to have shrinkage of the olfactory bulb. Participants in this study included 11 male adults (Mage = 58.18 yrs) with olfactory dysfunction (1 mild hyposmia, 3 severe hyposmia, & 6 anosmia). Exclusion criteria included history of nasal polyposis, neurologic or psychiatric disease, head trauma, or other ENT conditions. Baseline olfactory function was established with the orthonasal olfactory test developed by the Connecticut Chemosensory Clinical Research Center (CCCRC) which provides scores for butanol threshold and odor identification. OT or “olfactory rehabilitation” lasted 30 min/day for six months and consisted of presenting four odorants via a larynx bypass technique (details not presented). Olfactory lobe volume was measured at baseline and following OT using a manual segmentation method of images obtained from a structural MRI.
Data analysis revealed that olfactory bulb volume was significantly larger following OT. Similarly, CCCRC scores increased for both odor threshold detection and odor identification, such that no participant remained in the anosmic group. In summary, patients more than five years post-laryngectomy responded to OT with both improved olfactory functioning and increased olfactory bulb size. Study strengths include a unique population, long duration of training, and use of structural MRI to measure the olfactory bulb before and following OT. Study limitations include small sample size, lack of appropriate control groups (e.g., laryngectomy group without OT, healthy controls), unclear description of the larynx bypass technique, and lack of adherence data.
-
16.
Rezaeyan et al. (2022) – Neuroimaging Study (Structural)
In a three-group pre-post experimental design, Rezaeyan et al. (2022) examined the effect of OT on brain morphology and olfactory function while using four fixed odorants versus changing odorants periodically in patients with post-traumatic olfactory dysfunction. The study included 25 total patients (Mage = 28.24 yrs; 20 – 45 years old) each with a head injury within two years, diagnosis of anosmia, and lack of any diseases or disorders that may be associated with olfactory dysfunction. All participants received pre-post MRI as well as Sniffin’ Sticks testing. The control group (n = 9) received no OT, the classic OT (COT; n = 9) received the same four odorants over 16 weeks, and the modified OT (MOT; n = 7) received four new odorants every four weeks over 16 weeks. The OT was performed 2x/day for 10 s per odorant by smelling a brown glass jar (50 mL) with 1 mL of odorant on a cotton pad with a daily diary to document training. The COT and MOT began with rose, eucalyptus, lemon, and clove, and the MOT subsequently changed every four weeks.
Following OT, both the MOT and COT significantly increased in overall TDI testing (p < 0.001 & p = 0.002, respectively) and odor identification (p < 0.001 & p = 0.004, respectively); no significant difference between the MOT and COT groups on TDI scores emerged. The COT group was found to have increased cortical thickness in several areas including the cerebellum and frontal lobe. The MOT group had less cortical thickening in the right orbital frontal cortex and right insular when compared to the COT group. This study was primarily limited by its small sample size but was novel to include both a constant and modified odor group. Other limitations included lack of long-term follow-up and no way to control for severity of brain injury among the participants.
Cognitive & Neuroimaging Studies
-
17.
Chen et al. (2022) – Cognition and Neuroimaging Study (Structural & Functional Connectivity)
In a randomized two-group prospective controlled blinded study, Chen et al. (2022) examined the effect of OT on cognitive performance and fMRI measures of activation and grey matter volume during a passive odor detection setting. Participants with MCI were randomized either into an OT (n = 17, Mage = 72.7 yrs) or a sham-OT control group (n = 16, Mage = 70.6 yrs). Eligibility criteria included diagnosis of MCI, absence of acute or chronic sinonasal inflammation, absence of serious psychiatric, neurologic, or relevant medical illness, and evidence of structural brain damage on MRI (even if incidentally found). Baseline olfaction was established with the Sniffin’ Sticks test and did not differ across groups. All participants completed cognitive tests at baseline and posttest including Mini Mental State Examination, Wechsler Memory Scale-Revised, Wortschatz Vocabulary Test, Boston Naming Test, Nuremberg Age Inventory Vocabulary Test, CERAD verbal fluency test, and Beck Depression Inventory. OT consisted of presentation of four odorants from brown glass jars (rose, eucalyptus, lemon, cloves) for 15 s 2x/session at 2x/day over four months. The control group performed sham OT by sniffing from four odorless jars. fMRI measured brain activation during passive odor perception of a peach odorant delivered through an olfactometer at baseline and posttest.
Data analysis revealed no therapeutic benefit of OT on olfaction; however, there was a slight increase in global cognition in the OT group. Brain activation analysis showed a positive association between OT and activation in the left middle frontal gyrus and orbitofrontal cortex. In addition, changes in TDI score were positively associated with increases in frontal activation. However, no effect of OT on grey matter volume was observed. Study limitations included small sample size, lack of a healthy control group, and lack of adherence data. Interestingly, participants suffered from MCI of heterogeneous etiologies which might obscure any neuro-benefit of OT; moreover, the voxel-based analysis conducted here may not have been as sensitive as the analysis of cortical thickness conducted by Al Aïn et al. (2019).
Other Studies
-
18.
Hummel et al. (2018) – Other Study (Electro-Olfactogram (EOG))
In a two-group pre-post experimental design over 4–6 months, Hummel et al. (2018) examined the effects of OT on olfactory performance and electro-olfactogram (EOG) recordings in 38 patients (15 anosmic, Mage = 49 and 23 hyposmic, Mage = 55) with olfactory dysfunction (URTI or idiopathic etiology); for comparison, 27 normosmic adults (Mage = 49) were also administered EOG at one time but did not receive OT. Olfactory performance was measured at baseline and following OT using the Sniffin’ Sticks test. OT, which was completed by 23 of 38 (60.5%) patients, consisted of sniffing four odors (citronellal, eugenol, eucalyptol, phenyl ethyl alcohol) 2x/day for 10 s over 4–6 months. EOG recordings captured the response of neurons in the olfactory epithelium to chemical stimulation from odorants. In this study, EOG recordings captured responses to three odorants (phenylethyl alcohol (PEA), hydrogen sulfide, or CO2) to the left or right nostril. The olfactometer was inserted ~ 7 cm into the nasal cavity using endoscopy.
Analysis of baseline olfactory performance revealed a significant group effect for odor identification, odor discrimination, odor detection, and TDI; healthy controls performed the best and anosmia patients performed the worst. Following OT, the combined patient group showed significant improvement in odor identification but not in odor detection, odor discrimination, or TDI. Clinically relevant improvement was detected in eight of 23 (35%) smell impaired patients. All participants, including those with anosmia, showed EOG responses to PEA and hydrogen sulfide. As expected, response amplitude was more significant and more frequent in the healthy control group than in patients with olfactory dysfunction. Importantly, the patient group showed a significantly greater number of responses to PEA and hydrogen sulfide following OT. This improvement did not differ between those participants with and without clinically relevant improvement.
In summary, this study is unique in measuring the frequency and amplitude of olfactory receptor neuron responses both before and following OT. The EOG improvement following OT could be attributable to either an increase in olfactory receptor neurons or greater sensitivity of existing neurons due to repeated exposures. Study limitations include high attrition (39.5%) during OT and lack of cognitive assessment. Future research would benefit from a control group (no OT) with olfactory dysfunction and a healthy control group following OT.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vance, D.E., Del Bene, V.A., Kamath, V. et al. Does Olfactory Training Improve Brain Function and Cognition? A Systematic Review. Neuropsychol Rev 34, 155–191 (2024). https://doi.org/10.1007/s11065-022-09573-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-022-09573-0