Abstract
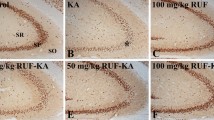
Temporal lobe epilepsy (TLE) is the most common form of partial and drug-resistant epilepsy, characterized by recurrent seizures originating from temporal lobe structures like the hippocampus. Hippocampal sclerosis and oxidative stress are two important factors in the pathogenesis of TLE that exacerbate epileptic seizures in this form of epilepsy. Recently, royal jelly (RJ) shown to have neuroprotective and antioxidant activities in several neurodegenerative models. Therefore, the aim of the present study was to investigate the pretreatment effect of RJ on epileptic seizures, hippocampal neuronal loss, and oxidative stress in the rat model of kainic acid (KA)-induced TLE. To this aim, 40 male Wistar rats weighing 200–250 g were divided into 4 groups, including control, vehicle, KA, and RJ + KA. Rats received RJ (150 mg/kg/day) for 14 days before induction of TLE with KA. Epileptic behaviors were evaluated according to Racine’s scale. Oxidative stress markers including, malondialdehyde (MDA), total oxidant status (TOS) and total antioxidant capacity (TAC) as well as neuronal loss in the CA1 region of the hippocampus (using Nissl staining) were evaluated in all groups. Our findings showed that RJ pretreatment significantly reduced the seizure score and increased the latency to the first seizure. RJ also reduced MDA and TOS while increasing TAC. In addition, RJ reversed neuronal damage in the hippocampal CA1 and CA3 areas. In conclusion, our results suggest that RJ has anticonvulsant and neuroprotective effects in KA induced TLE via its antioxidative properties.






Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References:
Tashakori-Miyanroudi M, Souresrafil A, Hashemi P, Ehsanzadeh SJ, Farrahizadeh M, Behroozi Z (2021) Prevalence of depression, anxiety, and psychological distress in patients with epilepsy during COVID-19: a systematic review. Epilepsy Behav 125:108410
Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR (2010) Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 51:883–890
Tashakori-Miyanroudi M, Ramazi S, Hashemi P, Nazari-Serenjeh M, Baluchnejadmojarad T, Roghani M (2022) Acetyl-L-carnitine exerts neuroprotective and anticonvulsant effect in kainate murine model of temporal lobe epilepsy. J Mol Neurosci 72:1224–1233
Vazifehkhah S, Ali MK, Babae JF, Hashemi P, Alireza MS, Nikbakht F (2020) Evaluation of the ameliorative effects of oral administration of metformin on epileptogenesis in the temporal lobe epilepsy model in rats. Life Sci 257:118066
Mojarad TB, Roghani M (2014) The anticonvulsant and antioxidant effects of berberine in kainate-induced temporal lobe epilepsy in rats. Basic Clin Neurosci 5:124–130
Lévesque M, Avoli M (2013) The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev 37:2887–2899
Rusina E, Bernard C, Williamson A (2021) The kainic acid models of temporal lobe epilepsy. eNeuro. https://doi.org/10.1523/ENEURO.0337-20.2021
Shin E-J, Jeong JH, Chung YH, Kim W-K, Ko K-H, Bach J-H, Hong J-S, Yoneda Y, Kim H-C (2011) Role of oxidative stress in epileptic seizures. Neurochem Int 59:122–137
Li Q, Chen N, Cai H, Tang Y, Zhou X, Huang Y, Gong M, Qin C, Wei X, Qi S (2018) Analysis of momordica charantia polysaccharide components and their effects on KA-induced oxidative stress and neuronal loss in the hippocampus of epileptic rats. World J Neurosci. https://doi.org/10.4236/wjns.2018.82011
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360:201–205
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Martinc B, Grabnar I, Vovk T (2014) Antioxidants as a preventive treatment for epileptic process: a review of the current status. Curr Neuropharmacol 12:527–550
Diniz TC, Silva JC, Lima-Saraiva SRGd, Ribeiro FPRdA, Pacheco AGM, de Freitas RM, Quintans-Júnior LJ, Quintans JdSS, Mendes RL, Almeida JRGdS (2015) The role of flavonoids on oxidative stress in epilepsy. Oxid Med Cell Longev. https://doi.org/10.1155/2015/171756
Varoglu AO, Yildirim A, Aygul R, Gundogdu OL, Sahin YN (2010) Effects of valproate, carbamazepine, and levetiracetam on the antioxidant and oxidant systems in epileptic patients and their clinical importance. Clin Neuropharmacol 33:155–157
Zeng L-H, Zhang H-D, Xu C-J, Bian Y-J, Xu X-J, Xie Q-M, Zhang R-H (2013) Neuroprotective effects of flavonoids extracted from licorice on kainate-induced seizure in mice through their antioxidant properties. J Zhejiang Univ Sci 14:1004–1012
Nikbakht F, Khadem Y, Haghani S, Hoseininia H, Sadat AM, Heshemi P, Jamali N (2019) Protective role of apigenin against Aβ 25–35 toxicity via inhibition of mitochondrial cytochrome c release. Basic Clin Neurosci 10:557–566
Buttstedt A, Moritz RF, Erler S (2014) Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol Rev 89:255–269
Ali AM, Kunugi H (2020) Royal jelly as an intelligent anti-aging agent—a focus on cognitive aging and alzheimer’s disease: a review. Antioxidants. https://doi.org/10.3390/antiox9100937
Honda Y, Fujita Y, Maruyama H, Araki Y, Ichihara K, Sato A, Kojima T, Tanaka M, Nozawa Y, Ito M (2011) Lifespan-extending effects of royal jelly and its related substances on the nematode Caenorhabditis elegans. PLoS ONE 6(8):e23527. https://doi.org/10.1371/journal.pone.0023527
Kheirdeh M, Koushkie Jahromi M, Brühl AB, Brand S (2022) The effect of exercise training and royal jelly on hippocampal cannabinoid-1-receptors and pain threshold in experimental autoimmune encephalomyelitis in rats as animal model of multiple sclerosis. Nutrients 14:4119
Ali AM, Kunugi H (2020) Apitherapy for Parkinson’s disease: a focus on the effects of propolis and royal jelly. Oxid Med Cell Longev. https://doi.org/10.1155/2020/1727142
Kawahata I, Xu H, Takahashi M, Murata K, Han W, Yamaguchi Y, Fujii A, Yamaguchi K, Yamakuni T (2018) Royal jelly coordinately enhances hippocampal neuronal expression of somatostatin and neprilysin genes conferring neuronal protection against toxic soluble amyloid-β oligomers implicated in Alzheimer’s disease pathogenesis. J Funct Foods 51:28–38
de Silva TGS, da Silva JRM, da Silva-Alves A, Britto LRG, Xavier GF, Sandoval MRL (2020) Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer’s disease. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e03281
Alaraj MD (2015) Royal jelly pretreatment can either protect or aggravate brain damage induced by hypoxia-ischemia in mice, depending on its dose. Int J Sci Basic Appl Res 19:338–346
El-Nekeety AA, El-Kholy W, Abbas NF, Ebaid A, Amra HA, Abdel-Wahhab MA (2007) Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon 50:256–269
Teixeira RR, de Souza AV, Peixoto LG, Machado HL, Caixeta DC, Vilela DD, Baptista NB, Franci CR, Espindola FS (2017) Royal jelly decreases corticosterone levels and improves the brain antioxidant system in restraint and cold stressed rats. Neurosci Lett 655:179–185
Bielefeld P, Sierra A, Encinas JM, Maletic-Savatic M, Anderson A, Fitzsimons CP (2017) A standardized protocol for stereotaxic intrahippocampal administration of kainic acid combined with electroencephalographic seizure monitoring in mice. Front Neurosci 11:1–9
Racine RJ (1972) Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Kim H-C, Choi D-Y, Jhoo W-K, Lee D-W, Koo C-H, Kim C (1997) Aspalatone, a new antiplatelet agent, attenuates the neurotoxicity induced by kainic acid in the rat. Life Sci 61:373–381
Chuang Y-C (2010) Mitochondrial dysfunction and oxidative stress in seizure-induced neuronal cell death. Acta Neurol Taiwan 19:3–15
Liang L, Ho Y, Patel M (2000) Mitochondrial superoxide production in kainate-induced hippocampal damage. Neurosci 101:563–570
Gittins R, Harrison PJ (2004) Neuronal density, size and shape in the human anterior cingulate cortex: a comparison of Nissl and NeuN staining. Brain Res Bull 63:155–160
Gorantla VR, Thomas SE, Millis RM (2019) Environmental enrichment and brain neuroplasticity in the kainate rat model of temporal lobe epilepsy. J Epilepsy Res 9:51–64
Zhen J-L, Chang Y-N, Qu Z-Z, Fu T, Liu J-Q, Wang W-P (2016) Luteolin rescues pentylenetetrazole-induced cognitive impairment in epileptic rats by reducing oxidative stress and activating PKA/CREB/BDNF signaling. Epilepsy Behav 57:177–184
Golechha M, Chaudhry U, Bhatia J, Saluja D, Arya DS (2011) Naringin protects against kainic acid-induced status epilepticus in rats: evidence for an antioxidant, anti-inflammatory and neuroprotective intervention. Biol Pharm Bull 34:360–365
Wu Z, Xu Q, Zhang L, Kong D, Ma R, Wang L (2009) Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem Res 34:1393–1400
Mouri G, Jimenez-Mateos E, Engel T, Dunleavy M, Hatazaki S, Paucard A, Matsushima S, Taki W, Henshall DC (2008) Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res 1213:140–151
Li J, O W, Li W, Jiang Z-G, Ghanbari HA (2013) Oxidative stress and neurodegenerative disorders. Int J Mol Sci 14:24438–24475
Maes M, Supasitthumrong T, Limotai C, Michelin AP, Matsumoto AK, de Oliveira SL, de Lima Pedrão JV, Moreira EG, Carvalho AF, Sirivichayakul S (2020) Increased oxidative stress toxicity and lowered antioxidant defenses in temporal lobe epilepsy and mesial temporal sclerosis: associations with psychiatric comorbidities. Mol Neurobiol 57:3334–3348
Kang S-M, Cha S-H, Ko J-Y, Kang M-C, Kim D, Heo S-J, Kim J-S, Heu MS, Kim Y-T, Jung W-K (2012) Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ Toxicol Pharmacol 34:96–105
Zaja-Milatovic S, Gupta RC, Aschner M, Montine TJ, Milatovic D (2008) Pharmacologic suppression of oxidative damage and dendritic degeneration following kainic acid-induced excitotoxicity in mouse cerebrum. Neurotoxicology 29:621–627
Pan Y, Xu J, Chen C, Chen F, Jin P, Zhu K, Hu CW, You M, Chen M, Hu F (2018) Royal jelly reduces cholesterol levels, ameliorates Aβ pathology and enhances neuronal metabolic activities in a rabbit model of Alzheimer’s disease. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2018.00050
Hattori N, Ohta S, Sakamoto T, Mishima S, Furukawa S (2011) Royal jelly facilitates restoration of the cognitive ability in trimethyltin-intoxicated mice. Evid Based Complement Alternat Med. https://doi.org/10.1093/ecam/nep029
Aslan A, Cemek M, Buyukokuroglu ME, Altunbas K, Bas O, Yurumez Y (2012) Royal jelly can diminish secondary neuronal damage after experimental spinal cord injury in rabbits. Food Chem Toxicol 50:2554–2559
Xue XF, Zhou JH, Wu LM, Fu LH, Zhao J (2009) HPLC determination of adenosine in royal jelly. Food Chem 115:715–719
Liu YJ, Chen J, Li X, Zhou X, Hu YM, Chu SF, Peng Y, Chen NH (2019) Research progress on adenosine in central nervous system diseases. CNS Neurosci Ther 25:899–910
Boison D (2012) Adenosine dysfunction in epilepsy. Glia 60:1234–1243
Hattori N, Nomoto H, Mishima S, Inagaki S, Goto M, Sako M, Furukawa S (2006) Identification of AMP N1-oxide in royal jelly as a component neurotrophic toward cultured rat pheochromocytoma PC12 cells. Biosci Biotechnol Biochem 70:897–906
Paulo MEFdVd, Silva JRMd, Alves AdS, Britto LRG, Xavier GF, Sandoval MRL (2020) Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer’s disease. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e03281
Pan Y, Xu J, Chen C, Chen F, Jin P, Zhu K, Hu CW, You M, Chen M, Hu F (2018) Royal jelly reduces cholesterol levels, ameliorates Aβ pathology and enhances neuronal metabolic activities in a rabbit model of alzheimer’s disease. Front Aging Neurosci 10:50
Ali AM, Kunugi H (2020) Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): a review on the effects of royal jelly, propolis, and bee pollen. Foods 9:1362
Almeer RS, Kassab RB, AlBasher GI, Alarifi S, Alkahtani S, Ali D, Abdel Moneim AE (2019) Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol Biol Rep 46:119–131
Inoue Y, Hara H, Mitsugi Y, Yamaguchi E, Kamiya T, Itoh A, Adachi T (2018) 4-Hydroperoxy-2-decenoic acid ethyl ester protects against 6-hydroxydopamine-induced cell death via activation of Nrf2-ARE and eIF2α-ATF4 pathways. Neurochem Int 112:288–296
Mohamed AA, Galal AA, Elewa YH (2015) Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochem 117:649–658
Goutman JD, Waxemberg MD, Doñate-Oliver F, Pomata PE, Calvo DJ (2003) Flavonoid modulation of ionic currents mediated by GABAA and GABAC receptors. Eur J Pharmacol 461:79–87
Subash S, Subramanian P (2009) Morin a flavonoid exerts antioxidant potential in chronic hyperammonemic rats: a biochemical and histopathological study. Mol Cell Biochem 327:153–161
Singh P, Singh D, Goel RK (2014) Phytoflavonoids: antiepileptics for the future. Int J Pharm Pharm Sci 6:51–66
Acknowledgements
The authors would like to acknowledge Dr. Kambiz Hassanzadeh for his valuable advice and input on this project.
Funding
The authors are thankful to the Vice Chancellor's office for Research Affairs and the Cellular & Molecular Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences for the Grants supporting this work. (The effect of Royal jelly on epileptic behaviors and hippocampal oxidative stress indices in experimental model of temporal lobe epilepsy; Grant/Award/Proposal number: IR.MUK.REC.1399.029).
Author information
Authors and Affiliations
Contributions
PH: Conceptualization, methodology, investigation, formal analysis, original draft preparation, reviewing & editing. ZV: and MRM: Investigation, formal analysis, writing and reviewing. EI: Supervision, reviewing & editing.
Corresponding authors
Ethics declarations
Competing Interests
Authors do not have any conflict of interest to be declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hashemi, P., Moloudi, M.R., Vahabzadeh, Z. et al. Anticonvulsant Effects of Royal Jelly in Kainic Acid-Induced Animal Model of Temporal Lobe Epilepsy Through Antioxidant Activity. Neurochem Res 48, 2187–2195 (2023). https://doi.org/10.1007/s11064-023-03897-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03897-w