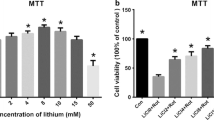
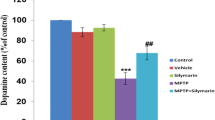
Abstract
We previously showed that kaempferol (KAE) could exert neuroprotective effects against PD. It has been demonstrated that abnormal autophagy plays a key role in the development of PD. Mitochondrial dysfunction, involved in the development of PD, can damage dopaminergic neurons. Whether the protective effects of KAE were exerted via regulating autophagy remains largely undefined, however. This study aimed to investigate whether KAE could protect dopaminergic neurons via autophagy and the underlying mechanisms using a MPTP/MPP+-stimulated PD model. Cell viability was detected by cell counting kit-8 (CCK-8) assay, and protein levels of autophagy mediators along with mTOR signaling pathway molecules were investigated by immunohistochemistry and Western blot analyses. The results showed that KAE could ameliorate the behavioral impairments of mice, reduce the loss of tyrosine hydroxylase (TH)-positive neurons in the substantia nigra pars compacta, and reduce α-synuclein (α-syn) levels. Furthermore, KAE upregulated levels of autophagy effector protein of Beclin-1 and autophagy microtubule associated protein of light chain 3 (LC3) in the substantia nigra (SN) while rescuing mitochondrial integrity, and downregulated levels of ubiquitin binding protein p62 and cleaved caspase-3, probably by decreasing the mammalian target of rapamycin (mTOR) signaling pathway. Further in vitro experiments demonstrated similar results. In conclusion, KAE exerts neuroprotective effects against PD potentially by promoting autophagy via inhibiting the mTOR signaling pathway.









Similar content being viewed by others
Data Availability
All data included in this study are available upon request by contact with the corresponding author.
References
Haque ME, Akther M, Jakaria M, Kim IS, Azam S, Choi DK (2020) Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease. Mov Disord 35(1):20–33. https://doi.org/10.1002/mds.27874
Raza C, Anjum R, Shakeel N (2019) Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci 226:77–90. https://doi.org/10.1016/j.lfs.2019.03.057
Trist BG, Hare DJ, Double KL (2019) Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 18(6):e13031. https://doi.org/10.1111/acel.13031
Öberg M, Fabrik I, Fabrikova D, Zehetner N, Härtlova A (2021) The role of innate immunity and inflammation in Parkinson´s disease. Scand J Immunol 93(5):e13022. https://doi.org/10.1111/sji.13022
Dorszewska J, Kowalska M, Prendecki M, Piekut T, Kozłowska J, Kozubski W (2021) Oxidative stress factors in Parkinson’s disease. Neural Regen Res 16(7):1383–1391. https://doi.org/10.4103/1673-5374.300980
Choi I, Zhang Y, Seegobin SP, Pruvost M, Wang Q, Purtell K, Zhang B, Yue Z (2020) Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun 11(1):1386. https://doi.org/10.1038/s41467-020-15119-w
Zhang K, Zhu S, Li J, Jiang T, Feng L, Pei J, Wang G, Ouyang L, Liu B (2021) Targeting autophagy using small-molecule compounds to improve potential therapy of Parkinson’s disease. Acta Pharm Sin B 11(10):3015–3034. https://doi.org/10.1016/j.apsb.2021.02.016
Yin Y, Sun G, Li E, Kiselyov K, Sun D (2017) ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev 34:3–14. https://doi.org/10.1016/j.arr.2016.08.008
Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D (2019) Synaptic, mitochondrial, and lysosomal dysfunction in Parkinson’s disease. Trends Neurosci 42(2):140–149. https://doi.org/10.1016/j.tins.2018.11.001
Du XY, Xie XX, Liu RT (2020) The role of α-synuclein oligomers in Parkinson’s disease. Int J Mol Sci. https://doi.org/10.3390/ijms21228645
Xilouri M, Brekk OR, Stefanis L (2016) Autophagy and alpha-synuclein: relevance to Parkinson’s disease and related synucleopathies. Mov Disord 31(2):178–192. https://doi.org/10.1002/mds.26477
Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci 40(3):151–166. https://doi.org/10.1016/j.tins.2017.01.002
Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Int J Mol Sci 20(3):728. https://doi.org/10.3390/ijms20030728
Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL (2013) ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol 15(7):741–750. https://doi.org/10.1038/ncb2757
Wang F, Wu H, Fan M, Yu R, Zhang Y, Liu J, Zhou X, Cai Y, Huang S, Hu Z, Jin X (2020) Sodium butyrate inhibits migration and induces AMPK-mTOR pathway-dependent autophagy and ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human bladder cancer cells. Faseb j 34(3):4266–4282. https://doi.org/10.1096/fj.201902626R
Cheng X, Yang YL, Yang H, Wang YH, Du GH (2018) Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway. Int Immunopharmacol 56:29–35. https://doi.org/10.1016/j.intimp.2018.01.002
Jin Y, Zhai Z, Jia H, Lai J, Si X, Wu Z (2021) Kaempferol attenuates diquat-induced oxidative damage and apoptosis in intestinal porcine epithelial cells. Food Funct 12(15):6889–6899. https://doi.org/10.1039/d1fo00402f
Kim TW, Lee SY, Kim M, Cheon C, Ko SG (2018) Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis 9(9):875. https://doi.org/10.1038/s41419-018-0930-1
Dabeek WM, Marra MV (2019) Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 11(10):2288. https://doi.org/10.3390/nu11102288
Yang YL, Cheng X, Li WH, Liu M, Wang YH, Du GH (2019) Kaempferol attenuates LPS-induced striatum injury in mice involving anti-neuroinflammation, maintaining BBB integrity, and down-regulating the HMGB1/TLR4 pathway. Int J Mol Sci 20(3):491. https://doi.org/10.3390/ijms20030491
Sloley BD, Urichuk LJ, Morley P, Durkin J, Shan JJ, Pang PK, Coutts RT (2000) Identification of kaempferol as a monoamine oxidase inhibitor and potential neuroprotectant in extracts of Ginkgo biloba leaves. J Pharm Pharmacol 52(4):451–459. https://doi.org/10.1211/0022357001774075
Huang WW, Tsai SC, Peng SF, Lin MW, Chiang JH, Chiu YJ, Fushiya S, Tseng MT, Yang JS (2013) Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int J Oncol 42(6):2069–2077. https://doi.org/10.3892/ijo.2013.1909
Gul A, Aimaiti M, Tuerxun T, Amat R, Reheman A, Zhang MF, Memtily N (2022) Study on the mechanism of Üstikuddus Sherbiti in ischemic cerebrovascular diseases: based on network pharmacology. Evid Based Complement Alternat Med 2022:5581864. https://doi.org/10.1155/2022/5581864
El-Kott AF, Bin-Meferij MM, Eleawa SM, Alshehri MM (2020) Kaempferol protects against cadmium chloride-induced memory loss and hippocampal apoptosis by increased intracellular glutathione stores and activation of PTEN/AMPK induced inhibition of Akt/mTOR signaling. Neurochem Res 45(2):295–309. https://doi.org/10.1007/s11064-019-02911-4
Yuan Y, Xia F, Gao R, Chen Y, Zhang Y, Cheng Z, Zhao H, Xu L (2022) Kaempferol mediated AMPK/mTOR signal pathway has a protective effect on cerebral ischemic-reperfusion injury in rats by inducing autophagy. Neurochem Res 47(8):2187–2197. https://doi.org/10.1007/s11064-022-03604-1
Inden M, Takagi A, Kitai H, Ito T, Kurita H, Honda R, Kamatari YO, Nozaki S, Wen X, Hijioka M, Kitamura Y, Hozumi I (2021) Kaempferol has potent protective and antifibrillogenic effects for α-synuclein neurotoxicity in vitro. Int J Mol Sci 22(21):11484. https://doi.org/10.3390/ijms222111484
Zhuang W, Cai M, Li W, Chen C, Wang Y, Lv E, Fu W (2020) Polyphenols from Toona sinensiss seeds alleviate neuroinflammation induced by 6-hydroxydopamine through suppressing p38 MAPK signaling pathway in a rat model of Parkinson’s disease. Neurochem Res 45(9):2052–2064. https://doi.org/10.1007/s11064-020-03067-2
Cai M, Zhuang W, Lv E, Liu Z, Wang Y, Zhang W, Fu W (2022) Kaemperfol alleviates pyroptosis and microglia-mediated neuroinflammation in Parkinson’s disease via inhibiting p38MAPK/NF-κB signaling pathway. Neurochem Int 152:105221. https://doi.org/10.1016/j.neuint.2021.105221
Chen C, Xia B, Tang L, Wu W, Tang J, Liang Y, Yang H, Zhang Z, Lu Y, Chen G, Yang Y, Zhao Y (2019) Echinacoside protects against MPTP/MPP+-induced neurotoxicity via regulating autophagy pathway mediated by Sirt1. Metab Brain Dis 34(1):203–212. https://doi.org/10.1007/s11011-018-0330-3
Kuribara H, Higuchi Y, Tadokoro S (1977) Effects of central depressants on rota-rod and traction performances in mice. Jpn J Pharmacol 27(1):117–126. https://doi.org/10.1254/jjp.27.117
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15(12):1257–1272. https://doi.org/10.1016/S1474-4422(16)30230-7
Blandini F, Armentero MT (2012) Animal models of Parkinson’s disease. FEBS J 279(7):1156–1166. https://doi.org/10.1111/j.1742-4658.2012.08491.x
Rahul, Siddique YH (2021) Neurodegenerative diseases and flavonoids: special reference to kaempferol. CNS Neurol Disord Drug Targets 20(4):327–342. https://doi.org/10.2174/1871527320666210129122033
Huang CH, Jan RL, Kuo CH, Chu YT, Wang WL, Lee MS, Chen HN, Hung CH (2010) Natural flavone kaempferol suppresses chemokines expression in human monocyte THP-1 cells through MAPK pathways. J Food Sci 75(8):H254-259. https://doi.org/10.1111/j.1750-3841.2010.01812.x
Daubner SC, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508(1):1–12. https://doi.org/10.1016/j.abb.2010.12.017
Limanaqi F, Biagioni F, Gambardella S, Familiari P, Frati A, Fornai F (2020) Promiscuous roles of autophagy and proteasome in neurodegenerative proteinopathies. Int J Mol Sci 21(8):3028. https://doi.org/10.3390/ijms21083028
Liu H, Ho PW, Leung CT, Pang SY, Chang E, Choi ZY, Kung MH, Ramsden DB, Ho SL (2021) Aberrant mitochondrial morphology and function associated with impaired mitophagy and DNM1L-MAPK/ERK signaling are found in aged mutant Parkinsonian LRRK2R1441G mice. Autophagy 17(10):3196–3220. https://doi.org/10.1080/15548627.2020.1850008
Kessel D (2019) Apoptosis, paraptosis and autophagy: death and survival pathways associated with photodynamic therapy. Photochem Photobiol 95(1):119–125. https://doi.org/10.1111/php.12952
Chu CT (2019) Mechanisms of selective autophagy and mitophagy: implications for neurodegenerative diseases. Neurobiol Dis 122:23–34. https://doi.org/10.1016/j.nbd.2018.07.015
Norris KL, Hao R, Chen LF, Lai CH, Kapur M, Shaughnessy PJ, Chou D, Yan J, Taylor JP, Engelender S, West AE, Lim KL, Yao TP (2015) Convergence of Parkin, PINK1, and α-synuclein on stress-induced mitochondrial morphological remodeling. J Biol Chem 290(22):13862–13874. https://doi.org/10.1074/jbc.M114.634063
Ashrafizadeh M, Tavakol S, Ahmadi Z, Roomiani S, Mohammadinejad R, Samarghandian S (2020) Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother Res 34(5):911–923. https://doi.org/10.1002/ptr.6577
Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP (2010) Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13(11):1763–1811. https://doi.org/10.1089/ars.2009.3074
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8(10):766–775. https://doi.org/10.1038/nrn2214
Perluigi M, Di Domenico F, Giorgi A, Schininà ME, Coccia R, Cini C, Bellia F, Cambria MT, Cornelius C, Butterfield DA, Calabrese V (2010) Redox proteomics in aging rat brain: involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J Neurosci Res 88(16):3498–3507. https://doi.org/10.1002/jnr.22500
Drake J, Sultana R, Aksenova M, Calabrese V, Butterfield DA (2003) Elevation of mitochondrial glutathione by gamma-glutamylcysteine ethyl ester protects mitochondria against peroxynitrite-induced oxidative stress. J Neurosci Res 74(6):917–927. https://doi.org/10.1002/jnr.10810
Liu ZQ, Yao GL, Zhai JM, Hu DW, Fan YG (2021) Kaempferol suppresses proliferation and induces apoptosis and DNA damage in human gallbladder cancer cells through the CDK4/CDK6/cyclin D1 pathway. Eur Rev Med Pharmacol Sci 25(3):1311–1321. https://doi.org/10.26355/eurrev_202102_24836
Liu GY, Sabatini DM (2020) mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 21(4):183–203. https://doi.org/10.1038/s41580-019-0199-y
Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20(7):1992–2003. https://doi.org/10.1091/mbc.e08-12-1249
Al-Bari M, Xu P (2020) Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann N Y Acad Sci 1467(1):3–20. https://doi.org/10.1111/nyas.14305
Che J, Liang B, Zhang Y, Wang Y, Tang J, Shi G (2017) Kaempferol alleviates ox-LDL-induced apoptosis by up-regulation of autophagy via inhibiting PI3K/Akt/mTOR pathway in human endothelial cells. Cardiovasc Pathol 31:57–62. https://doi.org/10.1016/j.carpath.2017.08.001
Schreiber KH, Arriola Apelo SI, Yu D, Brinkman JA, Velarde MC, Syed FA, Liao CY, Baar EL, Carbajal KA, Sherman DS, Ortiz D, Brunauer R, Yang SE, Tzannis ST, Kennedy BK, Lamming DW (2019) A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat Commun 10(1):3194. https://doi.org/10.1038/s41467-019-11174-0
Liu B, Deng X, Jiang Q, Li G, Zhang J, Zhang N, Xin S, Xu K (2020) Scoparone improves hepatic inflammation and autophagy in mice with nonalcoholic steatohepatitis by regulating the ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway in macrophages. Biomed Pharmacother 125:109895. https://doi.org/10.1016/j.biopha.2020.109895
Funding
This research project was supported by the National Natural Science Foundation of China (Grant Number 81870943), the Shandong Traditional Chinese Medicine Technology Development Project of China (Grant Number 2017-214), the Shandong Medical and Health Science and Technology Development Program of China (Grant Number 2015WS0063).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. [ZL] Investigation, Methodology, Writing—Original draft preparation; [WZ] Formal analysis, Writing—review and editing; [MC, ZW, and HW] Data curation, Methodology, Investigation; [YW] Supervision, Validation; [EL] Visualization, Investigation; [WF] Conceptualization, Supervision, Validation, Writing—reviewing and editing, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no known competing financial interests or personal relationships.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Zhuang, W., Cai, M. et al. Kaemperfol Protects Dopaminergic Neurons by Promoting mTOR-Mediated Autophagy in Parkinson’s Disease Models. Neurochem Res 48, 1395–1411 (2023). https://doi.org/10.1007/s11064-022-03819-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03819-2