Abstract
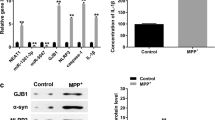
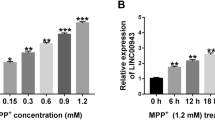
Long non-coding RNA (lncRNA) nuclear-enriched assembly transcript 1 (NEAT1) has been reported to be highly expressed in Parkinson’s disease (PD). However, the mechanism of NEAT1 in PD progression has not been fully elucidated. 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine injection (MPTP) was used to construct PD mouse models in vivo, and 1-methyl-4-phenyl pyridine (MPP+) was used to build PD cell models in vitro. Quantitative real-time polymerase chain reaction (qRT-PCR) was employed to test the expression of NEAT1, microRNA (miR)-212-3p and axis inhibition protein 1 (AXIN1). The viability, apoptosis and inflammation of cells were determined using cell counting kit 8 (CCK8) assay, flow cytometry and enzyme-linked immunosorbent assay (ELISA), respectively. Then, the protein levels of apoptosis-related markers and AXIN1 were measured by western blot (WB) analysis. Furthermore, dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay were performed to verify the interaction between miR-212-3p and NEAT1 or AXIN1. NEAT1 was upregulated in PD mouse models and cell models. Function experiments confirmed that NEAT1 knockdown could promote the viability, suppress the apoptosis and inflammation of MPP+-stimulated SK-N-SH cells to restrain PD progression. MiR-212-3p was downregulated in PD, and its inhibitor could reverse the suppression effect of NEAT1 knockdown on PD progression. Additionally, AXIN1 was a target of miR-212-3p, and its overexpression could invert the inhibition effect of miR-212-3p mimic on PD progression. Furthermore, AXIN1 expression was inhibited by NEAT1 silencing and promoted by NEAT1 overexpression, while these effect could be recovered by miR-212-3p inhibitor and mimic, respectively. Our results demonstrated that NEAT1 knockdown suppressed PD progression through regulating the miR-212-3p/AXIN1 pathway, indicating that NEAT1 might be a therapeutic target for neuroprotection in PD.






Similar content being viewed by others
References
Weintraub D, Stern MB (2005) Psychiatric complications in Parkinson disease. Am J Geriatr Psychiatry 13(10):844–851
Alvarez FJ (2016) Parkinson’s disease, antiparkinson medicines, and driving. Expert Rev Neurother 16(9):1023–1032
Postuma RB, Poewe W, Litvan I et al (2018) Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 33(10):1601–1608
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912
Chekani F, Bali V, Aparasu RR (2016) Quality of life of patients with Parkinson’s disease and neurodegenerative dementia: a nationally representative study. Res Soc Adm Pharm 12(4):604–613
Klemann C, Martens GJM, Poelmans G et al (2016) Validity of the MPTP-treated mouse as a model for Parkinson’s disease. Mol Neurobiol 53(3):1625–1636
Zhu J, Wang S, Liang Y et al (2018) Inhibition of microRNA-505 suppressed MPP+-induced cytotoxicity of SHSY5Y cells in an in vitro Parkinson’s disease model. Eur J Pharmacol 835:11–18
Wan P, Su W, Zhuo Y (2017) The role of long noncoding RNAs in neurodegenerative diseases. Mol Neurobiol 54(3):2012–2021
Peng T, Liu X, Wang J et al (2019) Long noncoding RNA HAGLROS regulates apoptosis and autophagy in Parkinson’s disease via regulating miR-100/ATG10 axis and PI3K/Akt/mTOR pathway activation. Artif Cells Nanomed Biotechnol 47(1):2764–2774
Cai L, Tu L, Li T et al (2019) Downregulation of lncRNA UCA1 ameliorates the damage of dopaminergic neurons, reduces oxidative stress and inflammation in Parkinson’s disease through the inhibition of the PI3K/Akt signaling pathway. Int Immunopharmacol 75:105734
Xu Y, Cao Z, Ding Y et al (2019) Long non-coding RNA NEAT1 alleviates acute-on-chronic liver failure through blocking TRAF6 mediated inflammatory response. Front Physiol 10:1503
Liu Q, Shi H, Yang J et al (2019) Long non-coding RNA NEAT1 promoted hepatocellular carcinoma cell proliferation and reduced apoptosis through the regulation of Let-7b-IGF-1R axis. Onco Targets Ther 12:10401–10413
Xie SP, Zhou F, Li J et al (2019) NEAT1 regulates MPP(+)-induced neuronal injury by targeting miR-124 in neuroblastoma cells. Neurosci Lett 708:134340
Liu Y, Lu Z (2018) Long non-coding RNA NEAT1 mediates the toxic of Parkinson’s disease induced by MPTP/MPP+ via regulation of gene expression. Clin Exp Pharmacol Physiol 45(8):841–848
Ni WJ, Leng XM (2016) miRNA-Dependent activation of mRNA translation. Microrna 5(2):83–86
Cui Y, Yi L, Zhao JZ et al (2017) Long noncoding RNA HOXA11-AS functions as miRNA sponge to promote the glioma tumorigenesis through targeting miR-140-5p. DNA Cell Biol 36(10):822–828
Chen S, Chen JZ, Zhang JQ et al (2019) Silencing of long noncoding RNA LINC00958 prevents tumor initiation of pancreatic cancer by acting as a sponge of microRNA-330–5p to down-regulate PAX8. Cancer Lett 446:49–61
Pichler S, Gu W, Hartl D et al (2017) The miRNome of alzheimer’s disease: consistent downregulation of the miR-132/212 cluster. Neurobiol Aging 50:167e1-267
Herrera-Espejo S, Santos-Zorrozua B, Alvarez-Gonzalez P et al (2019) A systematic review of microRNA expression as biomarker of late-onset alzheimer’s disease. Mol Neurobiol 56(12):8376–8391
Song Y, Liu Y, Chen X (2018) MiR-212 attenuates MPP(+)-induced neuronal damage by targeting KLF4 in SH-SY5Y cells. Yonsei Med J 59(3):416–424
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8(4):382–397
Binukumar BK, Shukla V, Amin ND et al (2015) Peptide TFP5/TP5 derived from Cdk5 activator P35 provides neuroprotection in the MPTP model of Parkinson’s disease. Mol Biol Cell 26(24):4478–4491
Qian C, Ye Y, Mao H et al (2019) Downregulated lncRNA-SNHG1 enhances autophagy and prevents cell death through the miR-221/222 /p27/mTOR pathway in Parkinson’s disease. Exp Cell Res 384(1):111614
Lyu L, Xiang W, Zhu JY et al (2019) Integrative analysis of the lncRNA-associated ceRNA network reveals lncRNAs as potential prognostic biomarkers in human muscle-invasive bladder cancer. Cancer Manag Res 11:6061–6077
Ke S, Yang Z, Yang F et al (2019) Long noncoding RNA NEAT1 aggravates abeta-induced neuronal damage by targeting miR-107 in alzheimer’s disease. Yonsei Med J 60(7):640–650
Li J, Quan H, Liu Q et al (2013) Alterations of axis inhibition protein 1 (AXIN1) in hepatitis B virus-related hepatocellular carcinoma and overexpression of AXIN1 induces apoptosis in hepatocellular cancer cells. Oncol Res 20(7):281–288
Salahshor S, Woodgett JR (2005) The links between axin and carcinogenesis. J Clin Pathol 58(3):225–236
Saeed M (2018) Genomic convergence of locus-based GWAS meta-analysis identifies AXIN1 as a novel Parkinson’s gene. Immunogenetics 70(9):563–570
Zhou L, Yang L, Li YJ et al (2018) MicroRNA-128 protects dopamine neurons from apoptosis and upregulates the expression of excitatory amino acid transporter 4 in parkinson’s disease by binding to AXIN1. Cell Physiol Biochem 51(5):2275–2289
Zhang G, Chen L, Liu J et al (2020) [HIF-1alpha/microRNA-128-3p axis protects hippocampal neurons from apoptosis via the Axin1-mediated Wnt/beta-catenin signaling pathway in Parkinson’s disease models. Aging 12(5):4067–4081
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, T., Zhang, Y., Liu, W. et al. LncRNA NEAT1 Regulates the Development of Parkinson’s Disease by Targeting AXIN1 Via Sponging miR-212-3p. Neurochem Res 46, 230–240 (2021). https://doi.org/10.1007/s11064-020-03157-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03157-1