Abstract
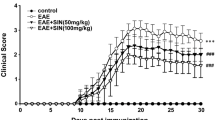
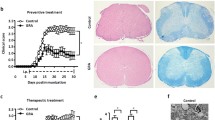
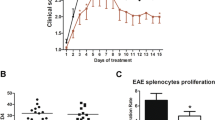
To assess the potential role of daphnetin, a clinically used anti-inflammatory agent, on the development of the inflammatory and neurodegenerative disease, we investigated its immune regulatory function in a murine model of experimental autoimmune encephalomyelitis (EAE). Significantly, lower levels of pro-inflammatory cytokines including interleukin (IL)-17, interferon-γ, Il6, Il12a, and Il23a were observed in brains of daphnetin-treated EAE mice, compared with those in control littermates. We also confirmed that daphnetin suppressed the production of IL-1β, IL-6, and tumor necrosis factor-α in lipopolysaccharide-stimulated mouse BV2 microglial cells. Mechanistically, heme oxygenase-1 (HO-1), a canonical anti-oxidant and anti-inflammatory factor, was found to be substantially induced by daphnetin treatment in BV2 cells. Also, a significantly higher level of HO-1, accompanied by a decreased level of malondialdehyde, was observed in daphnetin-treated EAE mice. More importantly, the deletion of HO-1 in BV2 microglia largely abrogated daphnetin-mediated inhibition of the inflammatory response. Together, our data demonstrate that daphnetin has an anti-inflammatory and neuroprotective role during the pathogenesis of EAE, which is partially at least, dependent on its regulation of HO-1.





Similar content being viewed by others
References
Nylander A, Hafler DA (2012) Multiple sclerosis. J Clin Invest 122:1180–1188
Hemmer B, Nessler S, Zhou D, Kieseier B, Hartung HP (2006) Immunopathogenesis and immunotherapy of multiple sclerosis. Nat Clin Pract Neurol 2:201–211
Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ (2014) Atlas of Multiple Sclerosis 2013: a growing global problem with widespread inequity. Neurology 83:1022–1024
Didonna A, Oksenberg JR (2015) Genetic determinants of risk and progression in multiple sclerosis. Clin Chim Acta 449:16–22
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, Clanet M, Comi G, Derfuss T, Fazekas F, Hartung HP, Havrdova E, Hemmer B, Kappos L, Liblau R, Lubetzki C, Marcus E, Miller DH, Olsson T, Pilling S, Selmaj K, Siva A, Sorensen PS, Sormani MP, Thalheim C, Wiendl H, Zipp F (2018) ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 24:96–120
Kurschus FC (2015) T cell mediated pathogenesis in EAE: molecular mechanisms. Biomed J 38:183–193
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8:913–919
Bo L, Dawson TM, Wesselingh S, Mork S, Choi S, Kong PA, Hanley D, Trapp BD (1994) Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol 36:778–786
van der Goes A, Brouwer J, Hoekstra K, Roos D, van den Berg TK, Dijkstra CD (1998) Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J Neuroimmunol 92:67–75
Van der Goes A, Wouters D, Van Der Pol SM, Huizinga R, Ronken E, Adamson P, Greenwood J, Dijkstra CD, De Vries HE (2001) Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J 15:1852–1854
Schreibelt G, van Horssen J, van Rossum S, Dijkstra CD, Drukarch B, de Vries HE (2007) Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res Rev 56:322–330
van Meeteren ME, Hendriks JJ, Dijkstra CD, van Tol EA (2004) Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochem Pharmacol 67:967–975
Ebers GC (2008) Environmental factors and multiple sclerosis. Lancet Neurol 7:268–277
Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG (2003) Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev 55:551–571
Hung SY, Liou HC, Kang KH, Wu RM, Wen CC, Fu WM (2008) Overexpression of heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Mol Pharmacol 74:1564–1575
Takata K, Kitamura Y, Kakimura J, Shibagaki K, Taniguchi T, Gebicke-Haerter PJ, Smith MA, Perry G, Shimohama S (2002) Possible protective mechanisms of heme oxygenase-1 in the brain. Ann NY Acad Sci 977:501–506
Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP (2007) Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest 117:438–447
van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, de Vries HE (2008) Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med 45:1729–1737
Liang SC, Ge GB, Liu HX, Zhang YY, Wang LM, Zhang JW, Yin L, Li W, Fang ZZ, Wu JJ, Li GH, Yang L (2010) Identification and characterization of human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of daphnetin. Drug Metab Dispos 38:973–980
Qi Z, Qi S, Gui L, Shen L, Feng Z (2016) Daphnetin protects oxidative stress-induced neuronal apoptosis via regulation of MAPK signaling and HSP70 expression. Oncol Lett 12:1959–1964
Lv H, Liu Q, Zhou J, Tan G, Deng X, Ci X (2017) Daphnetin-mediated Nrf2 antioxidant signaling pathways ameliorate tert-butyl hydroperoxide (t-BHP)-induced mitochondrial dysfunction and cell death. Free Radic Biol Med 106:38–52
Fukuda H, Nakamura S, Chisaki Y, Takada T, Toda Y, Murata H, Itoh K, Yano Y, Takata K, Ashihara E (2016) Daphnetin inhibits invasion and migration of LM8 murine osteosarcoma cells by decreasing RhoA and Cdc42 expression. Biochem Biophys Res Commun 471:63–67
Jimenez-Orozco FA, Rosales AA, Vega-Lopez A, Dominguez-Lopez ML, Garcia-Mondragon MJ, Maldonado-Espinoza A, Lemini C, Mendoza-Patino N, Mandoki JJ (2011) Differential effects of esculetin and daphnetin on in vitro cell proliferation and in vivo estrogenicity. Eur J Pharmacol 668:35–41
Tu L, Li S, Fu Y, Yao R, Zhang Z, Yang S, Zeng X, Kuang N (2012) The therapeutic effects of daphnetin in collagen-induced arthritis involve its regulation of Th17 cells. Int Immunopharmacol 13:417–423
Gao Q, Shan J, Di L, Jiang L, Xu H (2008) Therapeutic effects of daphnetin on adjuvant-induced arthritic rats. J Ethnopharmacol 120:259–263
Yao R, Fu Y, Li S, Tu L, Zeng X, Kuang N (2011) Regulatory effect of daphnetin, a coumarin extracted from Daphne odora, on the balance of Treg and Th17 in collagen-induced arthritis. Eur J Pharmacol 670:286–294
Yu WW, Lu Z, Zhang H, Kang YH, Mao Y, Wang HH, Ge WH, Shi LY (2014) Anti-inflammatory and protective properties of daphnetin in endotoxin-induced lung injury. J Agric Food Chem 62:12315–12325
Li M, Shi X, Chen F, Hao F (2017) Daphnetin inhibits inflammation in the NZB/W F1 systemic lupus erythematosus murine model via inhibition of NF-kappaB activity. Exp Ther Med 13:455–460
Liu J, Chen Q, Jian Z, Xiong X, Shao L, Jin T, Zhu X, Wang L (2016) Daphnetin protects against cerebral ischemia/reperfusion injury in mice via inhibition of TLR4/NF-kappaB signaling pathway. Biomed Res Int 2016:2816056
Liu ZY, Liu J, Zhao KL, Wang LK, Shi Q, Zuo T, Liu TY, Zhao L, Wang WX (2014) Protective effects of daphnetin on sodium taurocholateinduced severe acute pancreatitis in rats. Mol Med Rep 9:1709–1714
Wang D, Lu Z, Zhang H, Jin SF, Yang H, Li YM, Shi LY (2016) Daphnetin alleviates experimental autoimmune encephalomyelitis via regulating dendritic cell activity. CNS Neurosci Ther 22:558–567
Thompson AJ, Toosy AT, Ciccarelli O (2010) Pharmacological management of symptoms in multiple sclerosis: current approaches and future directions. Lancet Neurol 9:1182–1199
Jokubaitis VG, Gresle MM, Kemper DA, Doherty W, Perreau VM, Cipriani TL, Jonas A, Shaw G, Kuhlmann T, Kilpatrick TJ, Butzkueven H (2013) Endogenously regulated Dab2 worsens inflammatory injury in experimental autoimmune encephalomyelitis. Acta Neuropathol Commun 1:32
Yu W, Wang H, Ying H, Yu Y, Chen D, Ge W, Shi L (2014) Daphnetin attenuates microglial activation and proinflammatory factor production via multiple signaling pathways. Int Immunopharmacol 21:1–9
Zhang Y, Zhang LH, Chen X, Zhang N, Li G (2018) Piceatannol attenuates behavioral disorder and neurological deficits in aging mice via activating the Nrf2 pathway. Food Funct 9:371–378
Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ (2009) Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol 65:457–469
Steinman L (1996) Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85:299–302
Leung G, Sun W, Zheng L, Brookes S, Tully M, Shi R (2011) Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune encephalomyelitis mouse. Neuroscience 173:150–155
Fialkow L, Wang Y, Downey GP (2007) Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med 42:153–164
Mohamed MR, Emam MA, Hassan NS, Mogadem AI (2014) Umbelliferone and daphnetin ameliorate carbon tetrachloride-induced hepatotoxicity in rats via nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1 expression. Environ Toxicol Pharmacol 38:531–541
Kumar A, Jha S, Pattanayak SP (2016) Daphnetin ameliorates 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis through Nrf-2-Keap1 and NF-kappaB pathways. Biomed Pharmacother 82:439–448
Luchtman DW, Ellwardt E, Larochelle C, Zipp F (2014) IL-17 and related cytokines involved in the pathology and immunotherapy of multiple sclerosis: current and future developments. Cytokine Growth Factor Rev 25:403–413
Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G (2009) Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J Exp Med 206:1167–1179
Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad ARA (2015) Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 74:27–34
Wang Y, Marling SJ, Martino VM, Prahl JM, Deluca HF (2016) The absence of 25-hydroxyvitamin D-3-1 alpha-hydroxylase potentiates the suppression of EAE in mice by ultraviolet light. J Steroid Biochem Mol Biol 163:98–102
Samoilova EB, Horton JL, Chen Y (1998) Acceleration of experimental autoimmune encephalomyelitis in interleukin-10-deficient mice: roles of interleukin-10 in disease progression and recovery. Cell Immunol 188:118–124
Airas L, Nylund M, Rissanen E (2018) Evaluation of microglial activation in multiple sclerosis patients using positron emission tomography. Front Neurol 9:181
Berard JL, Wolak K, Fournier S, David S (2010) Characterization of relapsing-remitting and chronic forms of experimental autoimmune encephalomyelitis in C57BL/6 mice. Glia 58:434–445
Berard JL, Kerr BJ, Johnson HM, David S (2010) Differential expression of SOCS1 in macrophages in relapsing-remitting and chronic EAE and its role in disease severity. Glia 58:1816–1826
Bhasin M, Wu M, Tsirka SE (2007) Modulation of microglial/macrophage activation by macrophage inhibitory factor (TKP) or tuftsin (TKPR) attenuates the disease course of experimental autoimmune encephalomyelitis. BMC Immunol 8:10
Lee DS, Ko W, Yoon CS, Kim DC, Yun J, Lee JK, Jun KY, Son I, Kim DW, Song BK, Choi S, Jang JH, Oh H, Kim S, Kim YC (2014) KCHO-1, a novel antineuroinflammatory agent, inhibits lipopolysaccharide-induced neuroinflammatory responses through Nrf2-mediated heme oxygenase-1 expression in mouse BV2 microglia cells. Evid Based Complement Alternat Med 2014:357154
Acknowledgements
This work was supported by National Natural Scientific Funds (81770014, 81470210 and 81270066), the National Key Research and Development Program Project (2018YFC1705900), Natural Science Foundation of Zhejiang Province (LGF19H250002), Zhejiang Sci-Tech University scientific research fund (16042187-Y), and a project funded by the priority academic program development of Jiangsu higher education institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 37297 kb)
Supplementary file3 (MP4 133992 kb)
Supplementary file4 (MP4 11130 kb)
Supplementary file5 (MP4 106876 kb)
Rights and permissions
About this article
Cite this article
Wang, D., Zhu, B., Liu, X. et al. Daphnetin Ameliorates Experimental Autoimmune Encephalomyelitis Through Regulating Heme Oxygenase-1. Neurochem Res 45, 872–881 (2020). https://doi.org/10.1007/s11064-020-02960-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-02960-0