Abstract
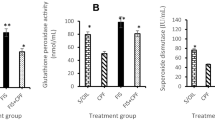
This study aimed to investigate the potential neurotoxic effects of aflatoxin B1 (AFB1) and the preventive effects of saffron. Male Balb-c mice received AFB1 (0.6 mg/kg/day intraperitoneally for 4 days), saffron infusion (90 mg styles/200 mL, ad libitum access for 2 weeks) or saffron infusion plus AFB1 (saffron treatment as previously plus 0.6 mg AFB1/kg/day intraperitoneally for the last 4 days). Control mice were intraperitoneally injected with DMSO:saline (1:1, v/v) during AFB1 treatment. Learning/memory was assessed by passive avoidance task. The activity of acetylcholinesterase [AChE, salt-(SS)/detergent-soluble(DS) isoforms], butyrylcholinesterase (BuChE, SS/DS isoforms), monoamine oxidase (MAO-A, MAO-B), the levels of lipid peroxidation (malondialdehyde, MDA) and reduced glutathione (GSH), were determined in whole brain (minus cerebellum) and cerebellum. We demonstrate for the first time that AFB1 administration impaired the memory of adult mice and decreased significantly whole brain AChE and BuChE activity, cerebellar AChE activity and cerebral GSH content. Moreover, MAO isoforms activity in whole brain, MAO-B activity in cerebellum and MDA levels of both tissues were significantly higher after AFB1 treatment. Pre-treatment with saffron prevented memory decline, activation of MAO-A and MAO-B in whole brain and cerebellum, respectively, and lipid peroxidation triggered by AFB1. Interestingly, the activity of AChE isoforms in whole brain, DS-AChE in cerebellum and GSH levels of both tissues were further significantly decreased in saffron +AFB1-treated mice compared with AFB1 group. Our findings support the neuroprotective efficacy of saffron against AFB1 in adult mice.



Similar content being viewed by others
References
Larsson P, Tjälve H (1993) Distribution and metabolism of aflatoxin B1 in the marmoset monkey (Callithrix jacchus). Carcinogenesis 14:1–6
Wogan GN, Edwards GS, Shank RC (1967) Excretion and tissue distribution of radioactivity from aflatoxin B1-14C in rats. Cancer Res 27:1729–1736
Oyelami OA, Maxwell SM, Adelusola KA, Aladekoma TA, Oyelese AO (1995) Aflatoxins in the autopsy brain tissue of children in Nigeria. Mycopathologia 132:35–38
Qureshi H, Hamid SS, Ali SS, Anwar J, Siddiqui AA, Khan NA (2015) Cytotoxic effects of aflatoxin B1 on human brain microvascular endothelial cells of the blood-brain barrier. Med Mycol 53:409–416
McLean M, Dutton MF (1995) Cellular interactions and metabolism of aflatoxin: an update. Pharmacol Ther 65:163–192
Chou MW, Chen W (1997) Food restriction reduces aflatoxin B1 (AFB1)-DNA adduct formation, AFB1-glutathione conjugation, and DNA damage in AFB1-treated male F344 rats and B6C3F1 mice. J Nutr 127:210–217
Wang CJ, Shiow SJ, Lin JK (1991) Effects of crocetin on the hepatotoxicity and hepatic DNA binding of aflatoxin B1 in rats. Carcinogenesis 12:459–462
Niranjan BG, Avadhani NG (1980) Tissue specificity of mitochondrial monooxygenase system for aflatoxin B1 activation. Biochem Biophys Res Commun 94:1021–1026
Singh N, Clausen J (1980) Different tissue responses of mixed function oxidases and detoxifying enzymes to aflatoxin B1 administration in the rat. Br J Exp Pathol 61:611–616
Chentanez T, Glinsukon T, Patchimasiri V, Klongprakit C, Chentanez V (1986) The effects of aflatoxin B1 given to pregnant rats on the liver, brain and the behaviour of their offspring. Nutr Rep Int 34:379–386
Kihara T, Matsuo T, Sakamoto M, Yasuda Y, Yamamoto Y, Tanimura T (2000) Effects of prenatal aflatoxin B1 exposure on behaviors of rat offspring. Toxicol Sci 53:392–399
Supriya Ch, Reddy PS (2015) Prenatal exposure to aflatoxin B1: developmental, behavioral, and reproductive alterations in male rats. Naturwissenschaften 102:26
Wangikar PB, Dwivedi P, Sharma AK, Sinha N (2004) Effect in rats of simultaneous prenatal exposure to ochratoxin A and aflatoxin B1. II. Histopathological features of teratological anomalies induced in fetuses. Birth Defects Res B 71:352–358
Bahey NG, Elaziz HO, Gadalla KK (2015) Toxic effect of aflatoxin B1 and the role of recovery on the rat cerebral cortex and hippocampus. Tissue Cell 47:559–566
Rawi SM, Waggas AM (2013) Impact of 90-Day oral dosing with naturally occurring aflatoxin mixture on male Sprague-Dawley rat neurochemistry and behavioral pattern. Middle East J Sci Res 14:228–238
Ikegwuonu FI (1983) The neurotoxicity of aflatoxin B1 in the rat. Toxicology 28:247–259
Egbunike GN, Ikegwuonu FI (1984) Effect of aflatoxicosis on acetylcholinesterase activity in the brain and adenohypophysis of the male rat. Neurosci Lett 52:171–174
Jayasekara S, Drown DB, Coulombe RA Jr, Sharma RP (1989) Alteration of biogenic amines in mouse brain regions by alkylating agents. I. Effects of aflatoxin B1 on brain monoamines concentrations and activities of metabolizing enzymes. Arch Environ Contam Toxicol 18:396–403
Kimbrough TD, Llewellyn GC, Weekley LB (1992) The effect of aflatoxin B1 exposure on serotonin metabolism: response to a tryptophan load. Metab Brain Dis 7:175–182
Weekley LB (1991) Aflatoxin B1 alters central and systemic tryptophan and tyrosine metabolism: influence of immunomodulatory drugs. Metab Brain Dis 6:19–32
Trebak F, Alaoui A, Alexandre D, El Ouezzani S, Anouar Y, Chartrel N, Magoul R (2015) Impact of aflatoxin B1 on hypothalamic neuropeptides regulating feeding behavior. Neurotoxicology 49:165–173
Gesing A, Karbownik-Lewinska M (2008) Protective effects of melatonin and N-acetylserotonin on aflatoxin B1-induced lipid peroxidation in rats. Cell Biochem Funct 26:314–319
Karabacak M, Eraslan G, Kanbur M, Sarıca ZS (2015) Effects of Tarantula cubensis D6 on aflatoxin-induced injury in biochemical parameters in rats. Homeopathy 104:205–210
Hussain S, Khan MZ, Khan A, Javed I, Asi MR (2009) Toxico-pathological effects in rats induced by concurrent exposure to aflatoxin and cypermethrin. Toxicon 53:33–41
Schmidt M, Betti G, Hensel A (2007) Saffron in phytotherapy: pharmacology and clinical uses. Wien Med Wochenschr 157:315–319
Bathaie SZ, Mousavi SZ (2010) New applications and mechanisms of action of saffron and its important ingredients. Crit Rev Food Sci Nutr 50:761–786
Chryssanthi DG, Lamari FN, Georgakopoulos CD, Cordopatis P (2011) A new validated SPE-HPLC method for monitoring crocetin in human plasma-application after saffron tea consumption. J Pharm Biomed Anal 55:563–568
Asai A, Nakano T, Takahashi M, Nagao A (2005) Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J Agric Food Chem 53:7302–7306
Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, Lamari FN (2006) Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem 54:8762–8768
Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M (2011) Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res 219:197–204
Linardaki ZI, Orkoula MG, Kokkosis AG, Lamari FN, Margarity M (2013) Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem Toxicol 52:163–170
Lautenschläger M, Sendker J, Hüwel S, Galla HJ, Brandt S, Düfer M, Riehemann K, Hensel A (2015) Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier. Phytomedicine 22:36–44
Ahmad AS, Ansari MA, Ahmad M, Saleem S, Yousuf S, Hoda MN, Islam F (2005) Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol Biochem Behav 81:805–813
Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H, Shoyama Y, Toda A, Eyanagi R, Soeda S (2007) Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta 1770:578–584
Pitsikas N, Sakellaridis N (2006) Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav Brain Res 173:112–115
Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, Khani M (2005) Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res 19:148–151
Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, Hejazi SS, Yousefi MH, Alimardani R, Jamshidi A, Rezazadeh SA, Yousefi A, Zare F, Moradi A, Vossoughi A (2010) A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology (Berl.) 207:637–643
Farokhnia M, Shafiee Sabet M, Iranpour N, Gougol A, Yekehtaz H, Alimardani R, Farsad F, Kamalipour M, Akhondzadeh S (2014) Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: a double-blind randomized clinical trial. Hum Psychopharmacol 29:351–359
Talaei A, Hassanpour Moghadam M, Sajadi Tabassi SA, Mohajeri SA (2015) Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: a randomized, double-blind, placebo-controlled, pilot clinical trial. J Affect Disord 174:51–56
Wang CJ, Hsu JD, Lin JK (1991) Suppression of aflatoxin B1-induced hepatotoxic lesions by crocetin (a natural carotenoid). Carcinogenesis 12:1807–1810
Kaneto H (1997) Learning/memory processes under stress conditions. Behav Brain Res 83:71–74
Otano A, García-Osta A, Ballaz S, Frechilla D, Del Río J (1999) Facilitation by 8-OH-DPAT of passive avoidance performance in rats after inactivation of 5-HT(1A) receptors. Br J Pharmacol 128:1691–1698
Das A, Dikshit M, Nath C (2005) Role of molecular isoforms of acetylcholinesterase in learning and memory functions. Pharmacol Biochem Behav 81:89–99
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ellman GL, Courtney KD, Andres JrV, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Lassiter TL, Barone S Jr, Padilla S (1998) Ontogenetic differences in the regional and cellular acetylcholinesterase and butyrylcholinesterase activity in the rat brain. Dev Brain Res 105:109–123
Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, Lamari FN (2009) Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res 198:352–358
Mahmood I, Neau SH, Mason WD (1994) An enzymatic assay for the MAO-B inhibitor selegiline in plasma. J Pharm Biomed Anal 12:895–899
Xu Y, Wang Z, You W, Zhang X, Li S, Barish PA, Vernon MM, Du X, Li G, Pan J, Ogle WO (2010) Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur Neuropsychopharmacol 20:405–413
Mokrasch LC, Teschke EJ (1984) Glutathione content of cultured cells and rodent brain regions: a specific fluorometric assay. Anal Biochem 140:506–509
Agag BI (2004) Mycotoxins in foods and feeds 1-Aflatoxins. Ass Univ Bull Environ Res 7:173–205 http://www.aun.edu.eg/env_enc/env%20mar/173-206.PDF. Accessed 16 Jan 2015
Steyn M, Pitout MJ, Purchase IFH (1971) A comparative study on aflatoxin B1 metabolism in mice and rats. Br J Cancer 25:291–297
Chen Z, Huang C, Ding W (2016) Z-Guggulsterone improves the scopolamine-induced memory impairments through enhancement of the BDNF signal in C57BL/6 J mice. Neurochem Res 41:3322–3332
Siuciak JA, McCarthy SA, Chapin DS, Martin AN (2008) Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 197:115–126
Ueda H, Sasaki K, Halder SK, Deguchi Y, Takao K, Miyakawa T, Tajima A (2017) Prothymosin alpha-deficiency enhances anxiety-like behaviors and impairs learning/memory functions and neurogenesis. J Neurochem 141:124–136
Wang ZM, Cai P, Liu QH, Xu DQ, Yang XL, Wu JJ, Kong LY, Wang XB (2016) Rational modification of donepezil as multifunctional acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Eur J Med Chem 123:282–297
Guillaumin S, Dahhaoui M, Caston J (1991) Cerebellum and memory: an experimental study in the rat using a passive avoidance conditioning test. Physiol Behav 49:507–511
Sacchetti B, Scelfo B, Strata P (2005) The cerebellum: synaptic changes and fear conditioning. Neuroscientist 11:217–227
Choi SJ, Jeong CH, Choi SG, Chun JY, Kim YJ, Lee J, Shin DH, Heo HJ (2009) Zeatin prevents amyloid beta-induced neurotoxicity and scopolamine-induced cognitive deficits. J Med Food 12:271–277
Jeon SJ, Kim B, Park HJ, Zhang J, Kwon Y, Kim DH, Ryu JH (2017) The ameliorating effect of 1-palmitoyl-2-linoleoyl-3-acetylglycerol on scopolamine-induced memory impairment via acetylcholinesterase inhibition and LTP activation. Behav Brain Res 324:58–65
Jiang B, Song L, Huang C, Zhang W (2016) P7C3 attenuates the scopolamine-induced memory impairments in C57BL/6 J mice. Neurochem Res 41:1010–1019
Jung WY, Kim H, Park HJ, Jeon SJ, Park HJ, Choi HJ, Kim NJ, Jang DS, Kim DH, Ryu JH (2016) The ethanolic extract of the Eclipta prostrata L. ameliorates the cognitive impairment in mice induced by scopolamine. J Ethnopharmacol 190:165–173
Lim DW, Son HJ, Um MY, Kim IH, Han D, Cho S, Lee CH (2016) Enhanced cognitive effects of demethoxycurcumin, a natural derivative of curcumin on scopolamine-induced memory impairment in mice. Molecules 21:E1022
U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER) (2005) Guidance for industry estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM078932.pdf. Accessed 6 Mar 2016
Drever BD, Riedel G, Platt B (2011) The cholinergic system and hippocampal plasticity. Behav Brain Res 221:505–514
Appleyard ME (1995) Acetylcholinesterase induces long-term potentiation in CA1 pyramidal cells by a mechanism dependent on metabotropic glutamate receptors. Neurosci Lett 190:25–28
Cometa MF, Lorenzini P, Fortuna S, Volpe MT, Meneguz A, Palmery M (2005) In vitro inhibitory effect of aflatoxin B1 on acetylcholinesterase activity in mouse brain. Toxicology 206:125–135
Arduini F, Errico I, Amine A, Micheli L, Palleschi G, Moscone D (2007) Enzymatic spectrophotometric method for aflatoxin B detection based on acetylcholinesterase inhibition. Anal Chem 79:3409–3415
Hansmann T, Sanson B, Stojan J, Weik M, Marty JL, Fournier D (2009) Kinetic insight into the mechanism of cholinesterasterase inhibition by aflatoxin B1 to develop biosensors. Biosens Bioelectron 24:2119–2124
Melo A, Monteiro L, Lima RM, Oliveira DM, Cerqueira MD, El-Bachá RS (2011) Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives. Oxid Med Cell Longev 2011:467180
Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C (2005) Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 16:577–586
Carrillo MC, Carnovale CE, Monti JA (1990) Effect of aflatoxin B1 treatment in vivo on the in vitro activity of hepatic and extrahepatic glutathione S-transferase. Toxicol Lett 50:107–116
Maurya BK, Trigun SK (2016) Fisetin modulates antioxidant enzymes and inflammatory factors to inhibit aflatoxin-B1 induced hepatocellular carcinoma in rats. Oxid Med Cell Longev 2016:1972793
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2:219–236
Lymperopoulou CD, Lamari FN (2015) Saffron safety in humans: lessons from the animal and clinical studies. Med Aromat Plants 4:e164. doi:10.4172/2167-0412.1000e164
Xi L, Qian Z, Du P, Fu J (2007) Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine 14:633–636
Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR, Pahlvan S (2011) Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol 667:222–229
Naghizadeh B, Mansouri MT, Ghorbanzadeh B, Farbood Y, Sarkaki A (2013) Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine 20:537–542
Geromichalos GD, Lamari FN, Papandreou MA, Trafalis DT, Margarity M, Papageorgiou A, Sinakos Z (2012) Saffron as a source of novel acetylcholinesterase inhibitors: molecular docking and in vitro enzymatic studies. J Agric Food Chem 60:6131–6138
De Monte C, Carradori S, Chimenti P, Secci D, Mannina L, Alcaro F, Petzer A, N’Da CI, Gidaro MC, Costa G, Alcaro S, Petzer JP (2014) New insights into the biological properties of Crocus sativus L.: chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur J Med Chem 82:164–171
Bandegi AR, Rashidy-Pour A, Vafaei AA, Ghadrdoost B (2014) Protective effects of Crocus sativus L. extract and crocin against chronic-stress induced oxidative damage of brain, liver and kidneys in rats. Adv Pharm Bull 4(Suppl. 2):493–499
Saleem S, Ahmad M, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Islam F (2006) Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J Med Food 9:246–253
Acknowledgements
Partial support from the University of Patras Research Committee is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable national guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Linardaki, Z.I., Lamari, F.N. & Margarity, M. Saffron (Crocus sativus L.) Tea Intake Prevents Learning/Memory Defects and Neurobiochemical Alterations Induced by Aflatoxin B1 Exposure in Adult Mice. Neurochem Res 42, 2743–2754 (2017). https://doi.org/10.1007/s11064-017-2283-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2283-z