Abstract
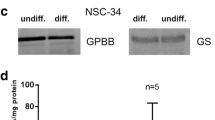
Glycogen is the main storage form of glucose in the brain. In contrast with previous beliefs, brain glycogen has recently been shown to play important roles in several brain functions. A fraction of metabolized glucose molecules are being shunted through glycogen before reentering the glycolytic pathway, a phenomenon known as the glycogen shunt. The significance of glycogen in astrocyte energetics is underlined by high activity of the glycogen shunt and the finding that inhibition of glycogen degradation, under some conditions leads to a disproportional increase in glycolytic activity, so-called glycolytic supercompensation. Glycogen phosphorylase, the key enzyme in glycogen degradation, is expressed in two different isoforms in brain, the muscle and the brain isoform. Recent studies have illustrated how these are differently regulated. In the present study, we investigate the role of the two isoforms in glycolytic supercompensation in cultured astrocytes with the expression of either one of the isoforms silenced by siRNA knockdown. When reintroducing glucose to glucose-starved astrocytes, glycolytic activity increased dramatically. Interestingly, the increase was 30% higher in astrocytes not expressing the muscle isoform of glycogen phosphorylase. Based on these results and previously published data we couple the muscle isoform of glycogen phosphorylase to glycolytic supercompensation and glycogen shunt activity, giving insights to the underlying mechanistic of these phenomena.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Brown AM (2004) Brain glycogen re-awakened. J Neurochem 89(3):537–552
Brown AM, Ransom BR (2015) Astrocyte glycogen as an emergency fuel under conditions of glucose deprivation or intense neural activity. Metab Brain Dis 30(1):233–239
Watanabe H, Passonneau JV (1973) Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. J Neurochem 20(6):1543–1554
Swanson RA (1992) Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can J Physiol Pharmacol 70:S138–S144
Brown AM et al (2005) Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res 79(1–2):74–80
Gibbs ME, Anderson DG, Hertz L (2006) Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 54(3):214–222
Sickmann HM et al (2009) Functional significance of brain glycogen in sustaining glutamatergic neurotransmission. J Neurochem 109(1):80–86
Suzuki A et al (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144(5):810–823
Obel LF et al (2012) Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Front Neuroenerg 4:3
Schmid H et al (2009) Expression of the brain and muscle isoforms of glycogen phosphorylase in rat heart. Neurochem Res 34(3):581–586
Newgard CB, Hwang PK, Fletterick RJ (1989) The family of glycogen phosphorylases: structure and functio. Crit Rev Biochem Mol Biol 24(1):69–99
Zhang Y et al (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34(36):11929–11947
Holtman IR et al (2015) Glia Open Access Database (GOAD): a comprehensive gene expression encyclopedia of glia cells in health and disease. Glia 63(9):1495–1506
Müller MS et al (2015) Isoform-selective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia 63(1):154–162
Dienel GA, Ball KK, Cruz NF (2007) A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J Neurochem 102(2):466–478
Walls AB et al (2009) Robust glycogen shunt activity in astrocytes: effects of glutamatergic and adrenergic agents. Neuroscience 158(1):284–292
Shulman RG, Rothman DL (2001) The “glycogen shunt” in exercising muscle: a role for glycogen in muscle energetics and fatigue. Proc Natl Acad Sci 98(2):457–461
Shulman RG, Hyder F, Rothman DL (2001) Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci 98(11):6417–6422
Hertz L et al (1982) Astrocytes in primary cultures. Neurosci Approached Cell Cult 1:175–186
Hertz L et al (1989) Preparation of primary cultures of mouse (rat) astrocytes. A dissection and tissue culture manual for the nervous system, pp 105–108
Hertz L, Peng L, Lai JC (1998) Functional studies in cultured astrocytes. Methods 16(3):293–310
Ferrick DA, Neilson A, Beeson C (2008) Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today 13(5):268–274
Waagepetersen HS, Westergaard N, Schousboe A (2000) The effects of isofagomine, a potent glycogen phosphorylase inhibitor, on glycogen metabolism in cultured mouse cortical astrocytes. Neurochem Int 36(4–5):435–440
Acknowledgements
The experimental work was supported by grants from the Hørslev and Augustinus Foundations to L.K.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jakobsen, E., Bak, L.K., Walls, A.B. et al. Glycogen Shunt Activity and Glycolytic Supercompensation in Astrocytes May Be Distinctly Mediated via the Muscle Form of Glycogen Phosphorylase. Neurochem Res 42, 2490–2494 (2017). https://doi.org/10.1007/s11064-017-2267-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2267-z