Abstract
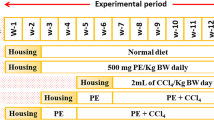
Acai offers health benefits associated with its high antioxidante capacity, phytochemical composition, nutritional and sensory value. Therefore, the objective of this study was to evaluate the protective effect of acai frozen pulp on carbon tetrachloride (CCl4)-induced damage via modulation of anti- and pro-inflammatory cytokines in rat brain tissue. The rats were treated via oral (gavage) daily with water or acai frozen pulp for 14 days at a dose of 7 μL/g. On the 15th day, the animals in each group received a single intraperitoneal injection of CCl4 in a dose of 3.0 mL/kg or the same volume of mineral oil. After 4 h, the animals were euthanized by decapitation and the cerebral cortex, hippocampus and cerebellum were dissected and homogenated to evaluate the levels of tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), interleukin 18 (IL-18), interleukin 6 (IL-6) and interleukin 10 (IL-10). Data were statistically analyzed by analysis of variance followed by the Tukey post hoc test. It was observed that CCl4 increased TNF-α, IL-1β and IL-18 levels in all brain tissues, and that acai frozen pulp was able to prevent this increase. IL-6 and IL-10 brain tissue levels remained unchanged during all treatments. CCl4 experimental model was suitable to investigate brain tissue anti and pro-inflammatory cytokines. Acai frozen pulp prevented an increase in IL-1β, IL-18 and TNF-α, while IL-6 and IL-10 levels remained unchanged. The precise pathway by which inflammation contribute to hepatic encephalopathy, as well as to how this pathway can be modulated, is still under investigation.





Similar content being viewed by others
References
Webster LT, Gabuzda GJ (1957) Ammonium uptake by the extremities and brain in hepatic coma. J Clin Investig 50:414–424. doi:10.1172/JCI103621
Häussinger D, Sies H (2013) Hepatic encephalopathy: clinical aspects and pathogenetic concept. Arch Biochem Biophys 536:97–100. doi:10.1016/j.abb.2013.04.013
McDermott WV (1958) The role of ammonia intoxication in hepatic coma. Bull NY Acad Med 34:357–365
Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Wendon JA (2011) Infection and systemic inflammation, not ammonia, are associated with grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol 54:640–649. doi:10.1016/j.jhep.2010.07.045
Acharya SK, Bhatia V, Sreenivas V, Khanal S, Panda SK (2009) Efficacy of l-ornithine l-aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology 136:2159–2168. doi:10.1053/j.gastro
Cichoż-lach H, Michalak A (2013) Current pathogenetic aspects of hepatic encephalopathy and noncirrhotic hyperammonemic encephalopathy. World J Gastroenterol 19:26–34. doi:10.3748/wjg.v19.i1.26
Butterworth RF (2000) Hepatic encephalopathy: a neuropsychiatric disorder involving multiple neurotransmitter systems. Curr Opin Neurol 6:721–727
Bémeur C, Butterworth RF (2013) Liver-brain proinflammatory signalling in acute liver failure: role in the pathogenesis of hepatic encephalopathy and brain edema. Metab Brain Dis 28:145–150. doi:10.1007/s11011-012-9361-3
Lachmann V, Görg B, Bidmon HJ, Keitel V, Häussinger D (2013) Precipitants of hepatic encephalopathy induce rapid astrocyte swelling in an oxidative stress dependent manner. Arch Biochem Biophys 536:143–151. doi:10.1016/j.abb.2013.05.004
Jimenez W, Clària J, Arroyo V, Rodés J (1992) Carbon tetrachloride induced cirrhosis in rats: an useful tool for investigating the patogenesis in chronic liver disease. J Gastroenterol Hepatol 7:90–97. doi:10.1111/j.1440-1746.1992.tb00940
Basu S (2003) Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 189:113–127. doi:10.1016/S0300-483X(03)00157-4
Tirkey N, Pilkhawl S, Kuhad A, Chopra K (2005) Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol 5:1–8. doi:10.1186/1471-2210-5-2
Rocha SW, de França ME, Rodrigues GB, Barbosa KP, Nunes AK, Pastor AF, Oliveira AG, Oliveira WH, Luna RL, Peixoto CA (2014) Diethylcarbamazine reduces chronic inflammation and fibrosis in carbon tetrachloride-(CCl4) induced liver injury in mice. Mediators Inflamm 2014:696383. doi:10.1155/2014/696383
Dai Q, Borenstein AR, Jackson JC, Larson EB (2006) Fruit and vegetable juices and Alzheimer’s disease: the Kame Project. Am J Med 119:751–759. doi:10.1016/j.amjmed.2006.03.045
Goodwin JS, Brodwick M (1995) Diet, aging, and cancer. Clin Geriatr Med 11:577–589
Heo HJ, Choi SJ, Choi SG, Shin DH, Lee JM, Lee CY (2008) Effects of banana, orange, and apple on oxidative stress-induced neuro-toxicity in PC12 cells. J Food Sci 73:28–32. doi:10.1111/j.1750-3841.2007.00632.x
Kohlmeier L, Simonsen N, Mottus K (1995) Dietary modifiers of carcinogenics. Environ Health Perspect 103:177–184. doi:10.1289/ehp.95103s8177
Silalahi J (2002) Anticancer and health protective properties of citrus fruit components. Asia Pac J Clin Nutr 11:79–84. doi:10.1046/j.1440-6047.2002.00271.x
Steinmetz KA, Potter JD (1996) Vegetables, fruits, and cancer prevention: a review. J Am Assoc 96:1027–1039. doi:10.1016/S0002-8223(96)00273-8
Schauss AG, Wu X, Prior RL, Ou B, Patel D, Huang D, Kanabick JP (2006) Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe oleraceae mart. (acai). J Agric Food Chem 54:8598–8603. doi:10.1021/jf060976g
Bramanti V, Tomassoni D, Bronzi D, Grasso S, Currò M, Avitabile M, Li Volsi G, Renis M, Ientile R, Amenta F, Avola R (2010) Alpha-lipoic acid modulates GFAP, vimentin, nestin, cyclin D1 and MAP-kinase espression in astroglial cell cultures. Neurochem Res 35:2070–2077. doi:10.1007/s11064-010-0256-6
Gabardo T, Pripolli CM, Andrade RB, Gemelli T, Lima JDO, Oliveira AS, Medeiros NS, Wannmacher C, Dani C, Funchal C (2015) Assessment of changes in energy metabolism parameters provoked by carbon tetrachloride in Wistar rats and the protective effect of white grape juice. Toxicol Rep 2:645–653. doi:10.1016/j.toxrep.2015.03.011
Grasso S, Bramanti V, Tomassoni D, Bronzi D, Malfa G, Traini E, Napoli M, Renis M, Amenta F, Avola R (2014) Effect of lipoic acid and α-glyceryl-phosphoryl-choline on astroglial cell proliferation and differentiation in primary culture. J Neurosci Res 92:86–94. doi:10.1002/jnr.23289
Kramer K, Packer L, Hoppe P (2001) R-alpha-lipoic acid. Nutraceuticals in health and disease prevention. Marcel Dekker Inc, New York, pp 129–164
Nair V, Bang WY, Schreckinger E, Andarwulan N, Cisneros-Zevallos L (2015) The protective role of ternatin anthocyanins and quercetin glycosides from Butterfly Pea (Clitoria ternatea Leguminosae) Blue flower petals against LPS-induced inflammation in macrophage cells. J Agric Food Chem. doi:10.1021/acs.jafc.5b00928
Menezes FS, Falcão DQ, de Mendonça Filho RFW, Silveira CS, Renno MN, Rodrigues VP, Moreira DL, Matheus ME, Fernandes PD, Kaplan MAC (2005) Chemical and pharmacological survey on Brazilian medicinal plants using ethnopharmacological information as a tool. Acta Hortic 675:89–95
EMBRAPA (BDPA) 2000. Available in: http://www.bdpa.cnptia.embrapa.br/busca?b=ad&id=346224&biblioteca=vazio&busca=autoria:%22H.%22&qFacets=autoria:%22H.%22&sort=&paginacao=t&paginaAtual=1. Accessed June 2014
Spada PD, de Souza GG, Bortolini GV, Henriques JA, Salvador M (2008) Antioxidant, mutagenic, and antimutagenic activity of frozen fruits. J Med Food 11:144–151. doi:10.1089/jmf.2007.598
Sanabria N, Sangronis E (2007) Characterization of the acai or manaca (Euterpe oleracea Mart.): a fruit of the Amazon. Arch Latinoam Nutr 57:94–98
Spada PDS, Dani C, Bortolini GV, Funchal C, Henriques JAP, Salvador M (2009) Frozen fruit pulp of Euterpe oleraceae Mart. (Acai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J Med Food 12:1084–1088. doi:10.1089/jmf.2008.0236
Xie C, Kang J, Li Z, Schauss AG, Badger TM, Nagarajan S, Wu T, Wu X (2012) The açaí flavonoid velutin is a potent anti-inflammatory agent: blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J Nutr Biochem 23:1184–1191. doi:10.1016/j.jnutbio.2011.06.013
Lowry OH, Rosebrouh NJ, Lewis-Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. JBC. 193:265–275
Sherlock S (1958) Pathogenesis and management of hepatic coma. Am J Med 24:805–813. doi:10.1001/jama.1959.03000270058013
Bobermin LD, Quincozes-Santos A, Guerra MC, Leite MC, Souza DO, Gonçalves CA, Gottfried C (2012) Resveratrol prevents ammonia toxicity in astroglial cells. PLoS ONE 7:e52164. doi:10.1371/journal.pone.0052164
Jayakumar AR, Bethea JR, Tong XY, Gomez J, Norenberg MD (2011) NF-κB in the mechanism of brain edema in acute liver failure: studies in transgenic mice. Neurobiol Dis 41:498–507. doi:10.1016/j.nbd.2010.10.021
Rivera-Espinosa L, Floriano-Sánchez E, Pedraza-Chaverrí J, Coballase-Urrutia E, Sampieri AI, Ortega-Cuellar D, Cárdenas-Rodríguez N, Carmona-Aparicio L (2013) Contributions of microdialysis to new alternative therapeutics for hepatic encephalopathy. Int J Mol Sci 14:16184–16206. doi:10.3390/ijms140816184
Sekhar MS, Unnikrishnan MK, Rodrigues GS, Mukhopadhyay C (2013) Synbiotic formulation of probiotic and lactulose combination for hepatic encephalopathy treatment: a realistic hope? Med Hypotheses 81:167–168. doi:10.1016/j.mehy.2013.05.016
Sharma P, Sharma BC (2013) Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis 28:313–320. doi:10.1007/s11011-013-9392-4
Staziaki PV, Marques CM, Delattre AM, De Paula Cioni B, Rufino M, Dos Santos FV, Licks F, Marroni NP, Ferraz AC (2013) Fish oil has beneficial effects on behavior impairment and oxidative stress in rats subjected to a hepatic encephalopathy model. CNS Neurol Disord Drug Targets 12:84–93. doi:10.2174/1871527311312010014
Joseph JA, Ayyappan UP, Sasidharan SR, Mutyala S, Goudar KS, Agarwal A (2014) Ameliorative effect of Phytocee™ Cool against carbon tetrachloride-induced oxidative stress. Pharmacogn Res 6:320–325. doi:10.4103/0974-8490.138284
Shin MO, Moon JO (2010) Effect of dietary supplementation of grape skin and seeds on liver fibrosis induced by dimethylnitrosamine in rats. Nutr Res Pract 4:369–374. doi:10.4162/nrp.2010.4.5.369
Khoshbaten M, Aliasgarzadeh A, Masnadi K, Farhang S, Tarzamani MK, Babaei H, Kiani J, Zaare M, Najafipoor F (2010) Grape seed extract to improve liver function in patients with nonalcoholic fatty liver change. Saudi J Gastroenterol 16:194–197. doi:10.4103/1319-3767.65197
Hazell AS, Butterworth RF (1999) Hepatic encephalopathy: an update of pathophysiologic mechanisms. Proc Soc Exp Biol Med 222:99–112
Coltart I, Tranah TH, Shawcross DL (2013) Inflammation and hepatic encephalopathy. Arch Biochem Biophys 536:189–196. doi:10.1016/j.abb.2013.03.016
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145. doi:10.1016/j.it.2007.01.005
Green HF, Nolan YM (2012) GSK-3 mediates the release of IL-1beta, TNF-alpha and IL-10 from cortical glia. Neurochem Int 61:666–671. doi:10.1016/j.neuint.2012.07.003
Hu D, Wan L, Chen M, Caudle Y, LeSage G, Li Q, Yin D (2014) Essential role of IL-10/STAT3 in chronic stress-induced immune suppression. Brain Behav Immun 36:118–127. doi:10.1016/j.bbi.2013.10.016
Tanabe K, Matsushima-Nishiwaki R, Yamaguchi S, Iida H, Dohi S, Kozawa O (2010) Mechanisms of tumor necrosis factor-alpha-induced interleukin-6 synthesis in glioma cells. J Neuroinflamm 7:16. doi:10.1186/1742-2094-7-16
de Rivero Vaccari JP, Dietrich WD, Keane RW (2014) Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab 34:369–375. doi:10.1038/jcbfm.2013.227
Bossu P, Ciaramella A, Salani F, Vanni D, Palladino I, Caltagirone C, Scapigliati G (2010) Interleukin-18, from neuroinflammation to Alzheimer’s disease. Curr Pharm Des 16:4213–4224. doi:10.2174/138161210794519147
Tranah TH, Vijay GK, Ryan JM, Shawcross DL (2013) Systemic inflammation and ammonia in hepatic encephalopathy. Metab Brain Dis 28:1–5. doi:10.1007/s11011-012-9370-2
Halim AB, El-Ahmady O, Hassab-Allah S, Abdel-Galil F, Hafez Y, Darwish A (1997) Biochemical effect of antioxidants on lipids and liver function in experimentally-induced liver damage. Ann Clin Biochem 34:656–663. doi:10.1177/000456329703400610
Sikander M, Malik S, Parveen K, Ahmad M, Yadav D, Hafeez ZB, Bansal M (2013) Hepatoprotective effect of Origanum vulgare in Wistar rats against carbon tetrachloride-induced hepatotoxicity. Protoplasma 250:483–493. doi:10.1007/s00709-012-0431-5
Yang X, Yang S, Guo Y, Jiao Y, Zhao Y (2013) Compositional characterisation of soluble apple polysaccharides, and their antioxidant and hepatoprotective effects on acute CCl4-caused liver damage in mice. Food Chem 138:1256–1264. doi:10.1016/j.foodchem.2012.10.030
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2014) Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol In Vitro 28:479–484. doi:10.1016/j.tiv.2014.01.006
Dani C, Oliboni LS, Umezu FM, Pasquali MAB, Salvador M, Moreira JCF, Henriques JAP (2009) Antioxidant and antigenotoxic activities of purple grape juice—organic and conventional—in adult rats. J Med Food 12:1111–1118. doi:10.1089/jmf.2008.0256
Kan S, Devi SA, Kawashima S (1991) Effect of vitamin E on the accumulation of fluorescent material in cultured cerebral cortical cells of mice. Exp Gerontol 26:365–374. doi:10.1016/0531-5565(91)90048-Q
Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C (2008) Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem 56:4855–4873. doi:10.1021/jf0735073
Iaderoza M, Baldini VLS, Draetta SE, Bovi MLA (1992) Anthocyanins from fruits of açaí (Euterpeoleracea, Mart) and juçara (Euterpeedulis Mart). Trop Sci 32:41–46
Graf D, Seifert S, Bub A, Fröhling B, Dold S, Unger F, Römpp A, Watzl B (2013) Anthocyanin-rich juice does not affect gut-associated immunity in Fischer rats. Mol Nutr Food Res 57:1753–1761. doi:10.1002/mnfr.201300022
Acknowledgments
This work was supported by research grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Centro Universitário Metodista – IPA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
de Souza Machado, F., Marinho, J.P., Abujamra, A.L. et al. Carbon Tetrachloride Increases the Pro-inflammatory Cytokines Levels in Different Brain Areas of Wistar Rats: The Protective Effect of Acai Frozen Pulp. Neurochem Res 40, 1976–1983 (2015). https://doi.org/10.1007/s11064-015-1693-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1693-z