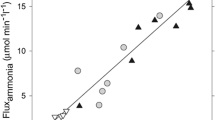
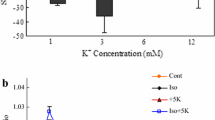
Abstract
Brain edema during hepatic encephalopathy or acute liver failure as well as following brain ischemia has a multifactorial etiology, but it is a dangerous and occasionally life-threatening complication because the brain is enclosed in the rigid skull. During ischemia the extracellular K+ concentration increases to very high levels, which when energy becomes available during reperfusion stimulate NKCC1, a cotransporter driven by the transmembrane ion gradients established by the Na+,K+-ATPase and accumulating Na+, K+ and 2 Cl− together with water. This induces pronounced astrocytic swelling under pathologic conditions, but NKCC1 is probably also activated, although to a lesser extent, during normal brain function. Redistribution of ions and water between extra- and intracellular phases does not create brain edema, which in addition requires uptake across the blood–brain barrier. During hepatic encephalopathy and acute liver failure a crucial factor is the close resemblance between K+ and NH4 + in their effects not only on NKCC1 and Na+,K+-ATPase but also on Na+,K+-ATPase-induced signaling by endogenous ouabains. These in turn activate production of ROS and nitrosactive agents which slowly sensitize NKCC1, explaining why cell swelling and brain edema generally are delayed under hyperammonemic conditions, although very high ammonia concentrations can cause immediate NKCC1 activation.











Similar content being viewed by others
References
Norenberg MD (1998) Astroglial dysfunction in hepatic encephalopathy. Metab Brain Dis 13:319–335
Vaquero J, Chung C, Blei AT (2003) Brain edema in acute liver failure. A window to the pathogenesis of hepatic encephalopathy. Ann Hepatol 2:12–22
Bosoi CR, Rose CF (2013) Brain edema in acute liver failure and chronic liver disease: similarities and differences. Neurochem Int 62:446–457
Rama Rao KV, Jayakumar AR, Norenberg MD (2014) Brain edema in acute liver failure: mechanisms and concepts. Metab Brain Dis (in press)
Gropman AL (2012) Patterns of brain injury in inborn errors of metabolism. Semin Pediatr Neurol 19:203–210
Ho ML, Rojas R, Eisenberg RL (2012) Cerebral edema. AJR Am J Roentgenol 199:W258–W273
Matsuoka Y, Hossmann KA (1982) Brain tissue osmolality after middle cerebral artery occlusion in cats. Exp Neurol 77:599–611
Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush B III, Reddy PV, Norenberg MD (2008) Na-K-Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem 283:33874–33882
Jayakumar AR, Panickar KS, Curtis KM, Tong XY, Moriyama M, Norenberg MD (2011) Na-K-Cl cotransporter-1 in the mechanism of cell swelling in cultured astrocytes after fluid percussion injury. J Neurochem 117:437–448
Jayakumar AR, Norenberg MD (2010) The Na-K-Cl Co-transporter in astrocyte swelling. Metab Brain Dis 25:31–38
Kelly T, Kafitz KW, Roderigo C, Rose CR (2009) Ammonium-evoked alterations in intracellular sodium and pH reduce glial glutamate transport activity. Glia 57:921–934
Kelly T, Rose CR (2010) Ammonium influx pathways into astrocytes and neurones of hippocampal slices. J Neurochem 115:1123–1136
Walz W, Hertz L (1984) Intense furosemide-sensitive potassium accumulation in astrocytes in the presence of pathologically high extracellular potassium levels. J Cereb Blood Flow Metab 4:301–304
Hertz L, Bender AS, Woodbury DM, White HS (1989) Potassium-stimulated calcium uptake in astrocytes and its potent inhibition by nimodipine. J Neurosci Res 22:209–215
Chow SY, Yen-Chow YC, White HS, Hertz L, Woodbury DM (1991) Effects of potassium on anion and cation contents of primary cultures of mouse astrocytes and neurons. Neurochem Res 16:1275–1283
White HS, Chow SY, Yen-Chow YC, Woodbury DM (1992) Effect of elevated potassium on the ion content of mouse astrocytes and neurons. Can J Physiol Pharmacol 70(Suppl):S263–S268
Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L (2013) Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res 38:472–485
Walz W, Kimelberg HK (1985) Differences in cation transport properties of primary astrocyte cultures from mouse and rat brain. Brain Res 340:333–340
Kimelberg HK, Frangakis MV (1985) Furosemide- and bumetanide-sensitive ion transport and volume control in primary astrocyte cultures from rat brain. Brain Res 361:125–134
Larsen BR, Assentoft M, Cotrina ML, Hua SZ, Nedergaard M, Kaila K, Voipio J, Macaulay N (2014) Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62:608–622
Pedersen SF, O’Donnell ME, Anderson SE, Cala PM (2006) Physiology and pathophysiology of Na+/H+ exchange and Na+ -K+ -2Cl− cotransport in the heart, brain, and blood. Am J Physiol Regul Integr Comp Physiol 291:R1–R25
Kurtz I, Balaban RS (1986) Ammonium as a substrate for Na+-K+-ATPase in rabbit proximal tubules. Am J Physiol 250:F497–F502
Rossi B, Gache C, Lazdunski M (1978) Specificity and interactions at the cationic sites of the axonal (Na+, K+)-activated adenosinetriphosphatase. Eur J Biochem 85:561–570
Skou JC, Esmann M (1992) The Na, K-ATPase. J Bioenerg Biomembr 24:249–261
Wall SM (1996) Ammonium transport and the role of the Na, K-ATPase. Miner Electrolyte Metab 22:311–317
Garçon DP, Lucena MN, Pinto MR, Fontes CF, McNamara JC, Leone FA (2012) Synergistic stimulation by potassium and ammonium of K+-phosphatase activity in gill microsomes from the crab Callinectes ornatus acclimated to low salinity: novel property of a primordial pump. Arch Biochem Biophys 530:55–63
Kala G, Kumarathasan R, Peng L, Leenen FH, Hertz L (2000) Stimulation of Na+, K+-ATPase activity, increase in potassium uptake, and enhanced production of ouabain-like compounds in ammonia-treated mouse astrocytes. Neurochem Int 36:203–211
Hannemann A, Flatman PW (2011) Phosphorylation and transport in the Na-K-2Cl cotransporters, NKCC1 and NKCC2A, compared in HEK-293 cells. PLoS ONE 6:e17992
Epstein FH, Silva P (1985) Na-K-Cl cotransport in chloride-transporting epithelia. Ann N Y Acad Sci 456:187–197
Dawson DC (1987) Cellular mechanisms for K transport across epithelial cell layers. Semin Nephrol 7:185–192
Hamann S, Herrera-Perez JJ, Zeuthen T, Alvarez-Leefmans FJ (2010) Cotransport of water by the Na+-K+-2Cl− cotransporter NKCC1 in mammalian epithelial cells. J Physiol 588:4089–4101
Marty S, Wehrlé R, Alvarez-Leefmans FJ, Gasnier B, Sotelo C (2002) Postnatal maturation of Na+, K+, 2Cl− cotransporter expression and inhibitory synaptogenesis in the rat hippocampus: an immunocytochemical analysis. Eur J Neurosci 15:233–245
Deisz RA, Lehmann TN, Horn P, Dehnicke C, Nitsch R (2011) Components of neuronal chloride transport in rat and human neocortex. J Physiol 589:1317–1347
Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K (2001) The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience 104:933–946
Yan YP, Dempsey RJ, Sun D (2001) Na-K-Cl- cotransporter in rat focal cerebral ischemia. J Cereb Blood Flow Metab 21:711–721
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278
Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS (2011) Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One 6:e25455
Hara H, Nagasawa H, Kogure K (1990) Nimodipine attenuates both ischaemia-induced brain oedema and mortality in a rat novel transient middle cerebral artery occlusion model. Acta Neurochir Suppl (Wien) 51:251–253
Campbell CA, Mackay KB, Patel S, King PD, Stretton JL, Hadingham SJ, Hamilton TC (1997) Effects of isradipine, an L-type calcium channel blocker on permanent and transient focal cerebral ischemia in spontaneously hypertensive rats. Exp Neurol 148:45–50
Deguchi K, Yamashita T, Abe K (2007) Prevention of neuronal damage by calcium channel blockers with antioxidative effects after transient focal ischemia in rats. Brain Res 1176:143–150
Ito Y, Araki N (2010) Calcium antagonists: current and future applications based on new evidence. Neuroprotective effect of calcium antagonists. Clin Calcium 20:83–88
Cai L, Du T, Song D, Li B, Hertz L, Peng L (2011) Astrocyte ERK phosphorylation precedes K+-induced swelling but follows hypotonicity-induced swelling. Neuropathology 31:250–264
Lang F, Ritter M, Wöll E, Weiss H, Häussinger D, Hoflacher J, Maly K, Grunicke H (1992) Altered cell volume regulation in ras oncogene expressing NIH fibroblasts. Pflugers Arch 420:424–427
Lytle C, Forbush B III (1992) The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J Biol Chem 267:25438–25443
Kurihara K, Moore-Hoon ML, Saitoh M, Turner RJ (1999) Characterization of a phosphorylation event resulting in upregulation of the salivary Na+-K+-2Cl− cotransporter. Am J Physiol 277:C1184–C1193
Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR (2006) Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 397(1):223–231
Delpire E, Gagnon KB (2008) SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J 409:321–331
Lothman E, Lamanna J, Cordingley G, Rosenthal M, Somjen G (1975) Responses of electrical potential, potassium levels, and oxidative metabolic activity of the cerebral neocortex of cats. Brain Res 88:15–36
Hansen AJ (1978) The extracellular potassium concentration in brain cortex following ischemia in hypo- and hyperglycemic rats. Acta Physiol Scand 102:324–329
Hansen AJ, Olsen CE (1980) Brain extracellular space during spreading depression and ischemia. Acta Physiol Scand 108:355–365
Zadunaisky JA, Curran PF (1963) Sodium fluxes in isolated frog brain. Am J Physiol 205:949–956
Bourke RS, Nelson KM (1972) Further studies on the K+- dependent swelling of primate cerebral cortex in vivo: the enzymatic basis of the K+- dependent transport of chloride. J Neurochem 19:663–685
Pappius HM, Elliott KA (1956) Water distribution in incubated slices of brain and other tissues. Can J Biochem Physiol 34:1007–1022
Bourke RS (1969) Studies of the development and subsequent reduction of swelling of mammalian cerebral cortex under isosmotic conditions in vitro. Exp Brain Res 8:232–248
Lund-Andersen H, Hertz L (1970) Effects of potassium content in brain-cortex slices from adult rats. Exp Brain Res 11:199–212
Boyle PJ, Conway EJ (1941) Potassium accumulation in muscle and associated changes. J Physiol 100:1–63
Bourke RS, Kimelberg HK, Nelson LR (1976) The effects of temperature and inhibitors on HCO3-stimulated swelling and ion uptake of monkey cerebral cortex. Brain Res 105:309–323
Moller M, Mollgård K, Lund-Andersen H, Hertz L (1974) Concordance between morphological and biochemical estimates of fluid spaces in rat brain cortex slices. Exp Brain Res 21:299–314
Zeuthen T, Macaulay N (2012) Cotransport of water by Na+-K+-2Cl− cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J Physiol 590:1139–1154
Schousboe A (1972) Development of potassium effects on ion concentrations and indicator spaces in rat brain-cortex slices during postnatal ontogenesis. Exp Brain Res 15:521–531
Cragoe EJ Jr, Gould NP, Woltersdorf OW Jr, Ziegler C, Bourke RS, Nelson LR, Kimelberg HK, Waldman JB, Popp AJ, Sedransk N (1982) Agents for the treatment of brain injury. 1. (Aryloxy)alkanoic acids. J Med Chem 25:567–579
Pond BB, Galeffi F, Ahrens R, Schwartz-Bloom RD (2004) Chloride transport inhibitors influence recovery from oxygen–glucose deprivation-induced cellular injury in adult hippocampus. Neuropharmacology 47:253–262
Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D (2009) Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 24:257–265
Khanna A, Kahle KT, Walcott BP, Gerzanich V, Simard JM (2014) Disruption of ion homeostasis in the neurogliovascular unit underlies the pathogenesis of ischemic cerebral edema. Transl Stroke Res 5:3–16
Lenart B, Kintner DB, Shull GE, Sun D (2004) Na-K-Cl cotransporter-mediated intracellular Na+ accumulation affects Ca2+ signaling in astrocytes in an in vitro ischemic model. J Neurosci 24:9585–9597
Walz W, Wuttke W, Hertz L (1984) Astrocytes in primary cultures: membrane potential characteristics reveal exclusive potassium conductance and potassium accumulator properties. Brain Res 292:367–374
Ransom CB, Sontheimer H (1995) Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes. J Neurophysiol 73:333–346
Hertz L, Kjeldsen CS (1985) Functional role of the potassium-induced stimulation of oxygen uptake in brain slices studied with cesium as a probe. J Neurosci Res 14:83–93
Newman EA (1989) Potassium conductance block by barium in amphibian Müller cells. Brain Res 498:308–314
Chen Y, McNeill JR, Hajek I, Hertz L (1992) Effect of vasopressin on brain swelling at the cellular level: do astrocytes exhibit a furosemide–vasopressin-sensitive mechanism for volume regulation? Can J Physiol Pharmacol 70(Suppl):S367–S373
Hertz L (1979) Inhibition by barbiturates of an intense net uptake of potassium into astrocytes. Neuropharmacology 18:629–632
Kostyuk PG (1984) Metabolic control of ionic channels in the neuronal membrane. Neuroscience 13:983–989
Xiong ZQ, Stringer JL (2000) Sodium pump activity, not glial spatial buffering, clears potassium after epileptiform activity induced in the dentate gyrus. J Neurophysiol 83:1443–1451
Hernández-Morales M, García-Colunga J (2009) Effects of nicotine on K+ currents and nicotinic receptors in astrocytes of the hippocampal CA1 region. Neuropharmacology 56:975–983
Hertz L, Xu J, Song D, Du T, Li B, Yan E, Peng L (2014) Astrocytic glycogenolysis: mechanisms and functions. Metab Brain Dis (in press)
Hertz L, Xu J, Peng L (2014) Glycogenolysis and purinergic signaling. In: Parpura V, Schousboe A, Verkhratsky A (ed) Glutamate and ATP at interface of metabolism and signaling in the brain. Springer, Berlin (in press)
Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M (2012) Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci Signal 5:ra26
Rangroo Thrane V, Thrane AS, Wang F, Cotrina ML, Smith NA, Chen M, Xu Q, Kang N, Fujita T, Nagelhus EA, Nedergaard M (2013) Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med 19:1643–1648
Himwich HE, Bernstein AO, Fazekas JF, Herrlich HC, Rich E (1942) The metabolic effects of potassium, temperature, methylene blue and paraphenylenediamine on infant and adult brain. Am J Physiol 137:327–330
Holtzman D, Olson J, Zamvil S, Nguyen H (1982) Maturation of potassium-stimulated respiration in rat cerebral cortical slices. J Neurochem 39:274–276
Hertz L, Schou M (1962) Univalent cations and the respiration of brain-cortex slices. Biochem J 85:93–104
Dickens F, Greville GD (1935) The metabolism of normal and tumour tissue: Neutral salt effects. Biochem J 29:1468–1483
Badar-Goffer RS, Ben-Yoseph O, Bachelard HS, Morris PG (1992) Neuronal-glial metabolism under depolarizing conditions. A 13C-n.m.r. study. Biochem J 282:225–230
Hertz L, Peng L, Kjeldsen CC, O’Dowd BS, Dienel GA (2004) Ion, transmitter and drug effects on energy metabolism in astrocytes. In: Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 435–460
Ashford CA (1934) Glycolysis in brain tissue. Biochem J 28:2229–2236
Hertz L (1966) Neuroglial localization of potassium and sodium effects on respiration in brain. J Neurochem 13:1373–1387
Hertz L, Dittmann L, Mandel P (1973) K+ induced stimulation of oxygen uptake in cultured cerebral glial cells. Brain Res 60:517–520
Hertz E, Hertz L (1979) Polarographic measurement of oxygen uptake by astrocytes in primary cultures using the tissue-culture flask as the respirometer chamber. In Vitro 15:429–436
Allert N, Köller H, Siebler M (1998) Ammonia-induced depolarization of cultured rat cortical astrocytes. Brain Res 782:261–270
Stephan J, Haack N, Kafitz KW, Durry S, Koch D, Hochstrate P, Seifert G, Steinhäuser C, Rose CR (2012) Kir4.1 channels mediate a depolarization of hippocampal astrocytes under hyperammonemic conditions in situ. Glia 60:965–978
Aickin CC, Deisz RA, Lux HD (1982) Ammonium action on post-synaptic inhibition in crayfish neurones: implications for the mechanism of chloride extrusion. J Physiol 329:319–339
Moser H (1987) Electrophysiological evidence for ammonium as a substitute for potassium in activating the sodium pump in a crayfish sensory neuron. Can J Physiol Pharmacol 65:141–145
Sørensen M, Keiding S (2007) New findings on cerebral ammonia uptake in HE using functional 13N-ammonia PET. Metab Brain Dis 22:277–284
Norenberg MD (1977) A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy. Progression and reversal of the disorder. Lab Invest 36:618–627
Traber PG, Dal Canto M, Ganger DR, Blei AT (1987) Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology 7:1272–1277
Swain MS, Blei AT, Butterworth RF, Kraig RP (1991) Intracellular pH rises and astrocytes swell after portacaval anastomosis in rats. Am J Physiol 261:R1491–R1496
Takahashi H, Koehler RC, Brusilow SW, Traystman RJ (1991) Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats. Am J Physiol 261:H825–H829
Martinez-Hernandez A, Bell KP, Norenberg MD (1977) Glutamine synthetase: glial localization in brain. Science 195:1356–1358
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161:303–310
Anlauf E, Derouiche A (2013) Glutamine synthetase as an astrocytic marker: its cell type and vesicle localization. Front Endocrinol (Lausanne) 4:144
Blei AT, Olafsson S, Therrien G, Butterworth RF (1994) Ammonia-induced brain edema and intracranial hypertension in rats after portacaval anastomosis. Hepatology 19:1437–1444
Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, Neary JT (1991) Ammonia-induced astrocyte swelling in primary culture. Neurochem Res 16:833–836
Dai H, Song D, Xu J, Li B, Hertz L, Peng L (2013) Ammonia-induced Na, K-ATPase/ouabain-mediated EGF receptor transactivation, MAPK/ERK and PI3K/AKT signaling and ROS formation cause astrocyte swelling. Neurochem Int 63:610–625
Ganz R, Swain M, Traber P, DalCanto M, Butterworth RF, Blei AT (1989) Ammonia-induced swelling of rat cerebral cortical slices: implications for the pathogenesis of brain edema in acute hepatic failure. Metab Brain Dis 4:213–223
Jayakumar AR, Valdes V, Norenberg MD (2011) The Na-K-Cl cotransporter in the brain edema of acute liver failure. J Hepatol 54:272–278
Zielińska M, Hilgier W, Law RO, Goryński P, Albrecht J (1999) Effects of ammonia in vitro on endogenous taurine efflux and cell volume in rat cerebrocortical minislices: influence of inhibitors of volume-sensitive amino acid transport. Neuroscience 91:631–638
Back A, Tupper KY, Bai T, Chiranand P, Goldenberg FD, Frank JI, Brorson JR (2011) Ammonia-induced brain swelling and neurotoxicity in an organotypic slice model. Neurol Res 33:1100–1108
Xue Z, Li B, Gu L, Hu X, Li M, Butterworth RF, Peng L (2010) Increased Na, K-ATPase alpha2 isoform gene expression by ammonia in astrocytes and in brain in vivo. Neurochem Int 57:395–403
Haas M, Wang H, Tian J, Xie Z (2002) Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem 277:18694–18702
Ferrari P, Ferrandi M, Valentini G, Bianchi G (2006) Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+- ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 290:R529–R535
Bai Y, Morgan EE, Giovannucci DR, Pierre SV, Philipson KD, Askari A, Liu L (2013) Different roles of the cardiac Na+/Ca2+-exchanger in ouabain-induced inotropy, cell signaling, and hypertrophy. Am J Physiol Heart Circ Physiol 304:H427–H435
Hawes BE, Luttrell LM, van Biesen T, Lefkowitz RJ (1996) Phosphatidylinositol 3-kinase is an early intermediate in the G beta gamma-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem 271:12133–12136
Liu L, Ivanov AV, Gable ME, Jolivel F, Morrill GA, Askari A (2011) Comparative properties of caveolar and noncaveolar preparations of kidney Na+/K+-ATPase. Biochemistry 50:8664–8673
Nakata S, Tsutsui M, Shimokawa H, Tamura M, Tasaki H, Morishita T, Suda O, Ueno S, Toyohira Y, Nakashima Y, Yanagihara N (2005) Vascular neuronal NO synthase is selectively upregulated by platelet-derived growth factor: involvement of the MEK/ERK pathway. Arterioscler Thromb Vasc Biol 25:2502–2508
Gan XT, Hunter JC, Huang C, Xue J, Rajapurohitam V, Javadov S, Karmazyn M (2012) Ouabain increases iNOS-dependent nitric oxide generation which contributes to the hypertrophic effect of the glycoside: possible role of peroxynitrite formation. Mol Cell Biochem 363:323–333
Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z (2000) Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem 275:27838–27844
Semplicini A, Serena L, Valle R, Ceolotto G, Felice M, Fontebasso A, Pessina AC (1995) Ouabain-inhibiting activity of aldosterone antagonists. Steroids 60:110–113
Tian J, Shidyak A, Periyasamy SM, Haller S, Taleb M, El-Okdi N, Elkareh J, Gupta S, Gohara S, Fedorova OV, Cooper CJ, Xie Z, Malhotra D, Bagrov AY, Shapiro JI (2009) Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension 54:1313–1320
Ernest NJ, Sontheimer H (2007) Extracellular glutamine is a critical modulator for regulatory volume increase in human glioma cells. Brain Res 1144:231–238
Tarbit I, Perry EK, Perry RH, Blessed G, Tomlinson BE (1980) Hippocampal free amino acids in Alzheimer’s disease. J Neurochem 35:1246–1249
Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD (2002) Neuronal and glial metabolite content of the epileptogenic human hippocampus. Ann Neurol 52:635–642
Mangia S, Tkác I, Gruetter R, Van De Moortele PF, Giove F, Maraviglia B, Uğurbil K (2006) Sensitivity of single-voxel 1H-MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magn Reson Imaging 24:343–348
Zhang NH, Laake J, Nagelhus E, Storm-Mathisen J, Ottersen OP (1991) Distribution of glutamine-like immunoreactivity in the cerebellum of rat and baboon (Papio anubis) with reference to the issue of metabolic compartmentation. Anat Embryol (Berl) 184:213–223
Albrecht J, Zielińska M, Norenberg MD (2010) Glutamine as a mediator of ammonia neurotoxicity: a critical appraisal. Biochem Pharmacol 80:1303–1308
Huang R, Kala G, Murthy RK, Hertz L (1994) Effects of chronic exposure to ammonia on glutamate and glutamine interconversion and compartmentation in homogeneous primary cultures of mouse astrocytes. Neurochem Res 19:257–265
Pichili VB, Rao KV, Jayakumar AR, Norenberg MD (2007) Inhibition of glutamine transport into mitochondria protects astrocytes from ammonia toxicity. Glia 55:801–809
Schousboe A, Hertz L, Svenneby G, Kvamme E (1979) Phosphate activated glutaminase activity and glutamine uptake in primary cultures of astrocytes. J Neurochem 32:943–950
Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Huang R, Peng L, Hertz L (1993) Glutamate and glutamine metabolism and compartmentation in astrocytes. Dev Neurosci 15:359–366
Rama Rao KV, Jayakumar AR, Norenberg MD (2003) Induction of the mitochondrial permeability transition in cultured astrocytes by glutamine. Neurochem Int 43:517–523
Jayakumar AR, Rama Rao KV, Schousboe A, Norenberg MD (2004) Glutamine-induced free radical production in cultured astrocytes. Glia 46:296–301
Zielińska M, Popek M, Albrecht J (2014) Roles of changes in active glutamine transport in brain edema development during hepatic encephalopathy: an emerging concept. Neurochem Res 39:599–604
Ruszkiewicz J, Fręśko I, Hilgier W, Albrecht J (2013) Decrease of glutathione content in the prefrontal cortical mitochondria of rats with acute hepatic encephalopathy: prevention by histidine. Metab Brain Dis 28:11–14
Hertz L, Peng L, Dienel GA (2007) Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 27:219–249
Whitelaw BS, Robinson MB (2013) Inhibitors of glutamate dehydrogenase block sodium-dependent glutamate uptake in rat brain membranes. Front Endocrinol (Lausanne) 4:123
McKenna MC (2013) Glutamate pays its own way in astrocytes. Front Endocrinol (Lausanne) 4:191
de Simone G, Chinali M, Mureddu GF, Cacciatore G, Lucci D, Latini R, Masson S, Vanasia M, Maggioni AP, Boccanelli A, AREA-in-CHF Investigators (2011) Effect of canrenone on left ventricular mechanics in patients with mild systolic heart failure and metabolic syndrome: the AREA-in-CHF study. Nutr Metab Cardiovasc Dis 21:783–791
Derosa G, Bonaventura A, Bianchi L, Romano D, D’Angelo A, Fogari E, Maffioli P (2013) Effects of canrenone in patients with metabolic syndrome. Expert Opin Pharmacother 14:2161–2169
Finn AM, Brown R, Sadée W (1977) Tissue distribution of 3H-canrenoate potassium in rabbits. J Pharm Sci 66:275–277
Rose CF (2012) Ammonia-lowering strategies for the treatment of hepatic encephalopathy. Clin Pharmacol Ther 92:321–331
Kala G, Hertz L (2005) Ammonia effects on pyruvate/lactate production in astrocytes–interaction with glutamate. Neurochem Int 47:4–12
Acknowledgments
This study was supported by Grants No. 30670651 and No. 31171036 to LP from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In honor of Michael Norenberg.
Rights and permissions
About this article
Cite this article
Hertz, L., Peng, L. & Song, D. Ammonia, Like K+, Stimulates the Na+, K+, 2 Cl− Cotransporter NKCC1 and the Na+,K+-ATPase and Interacts with Endogenous Ouabain in Astrocytes. Neurochem Res 40, 241–257 (2015). https://doi.org/10.1007/s11064-014-1352-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1352-9