Abstract
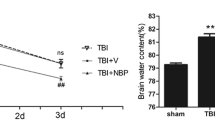
Naringin is neuroprotective in ischemia and other disease models. However, the effects of naringin are unknown after traumatic brain injury (TBI). The present study explored the role of naringin for neuroprotection in TBI rats. TBI was performed with the weight drop technique, and naringin was given orally at a dose of 100 mg/kg/day. The neurological scores, tissue edema, and oxidative stress/inflammation parameters [malondialdehyde (MDA), superoxide dismutase, nitric oxide, inducible nitric oxide synthase (iNOS), as well as interleukin-1β (IL-1β)] were measured. Compared to sham controls, TBI rats displayed obvious sensorimotor dysfunction, significant brain edema, and elevated oxidative and inflammatory molecules. Although a 7-day pre-treatment of naringin was unable to reverse these pathological changes, a 14-day continual treatment (7 days before and 7 days after the TBI) attenuated the increases in MDA and nitric oxide; enhanced the activation of superoxide dismutase; depressed the over-activation of iNOS; down-regulated the over-expression of IL-1β; and reduced the cortex edema. Additionally, the TBI-induced behavioral dysfunction was reduced. These results suggest that naringin treatment can attenuate cellular and histopathological alterations and improve the sensorimotor dysfunction of TBI rats, which may be partly due to the attenuation of oxidative and inflammatory damages.



Similar content being viewed by others
References
Corrigan JD, Selassie AW, Orman JA (2010) The epidemiology of traumatic brain injury. J Head Trauma Rehabil 25:72–80
Narayan RK, Michel ME, Ansell B et al (2002) Clinical trials in head injury. J Neurotrauma 19:503–557
Boto GR, Gomez PA, De la Cruz J et al (2005) Overview of the recent clinical trials in severe head injury and analysis of their therapeutic failure. Neurocirugia (Astur) 16:39–49
Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99:4–9
Cornelius C, Crupi R, Calabrese V et al (2013) Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal 19:836–853
Helmy A, De Simoni MG, Guilfoyle MR et al (2011) Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog Neurobiol 95:352–372
Jafarian-Tehrani M, Louin G, Royo NC et al (2005) 1400 W, a potent selective inducible nos inhibitor, improves histopathological outcome following traumatic brain injury in rats. Nitric Oxide 12:61–69
Haber M, Abdel Baki SG, Grin’kina NM et al (2013) Minocycline plus n-acetylcysteine synergize to modulate inflammation and prevent cognitive and memory deficits in a rat model of mild traumatic brain injury. Exp Neurol 249:169–177
Chen SF, Hung TH, Chen CC et al (2007) Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Sci 81:288–298
Potts MB, Koh SE, Whetstone WD et al (2006) Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx 3:143–153
Meffre D, Pianos A, Liere P et al (2007) Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology 148:2505–2517
Bramlett HM, Dietrich WD (2001) Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma 18:891–900
Wagner AK, Willard LA, Kline AE et al (2004) Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res 998:113–121
O’Connor CA, Cernak I, Vink R (2003) Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J Neurotrauma 20:533–541
Shahrokhi N, Haddad MK, Joukar S et al (2012) Neuroprotective antioxidant effect of sex steroid hormones in traumatic brain injury. Pak J Pharm Sci 25:219–225
Ma J, Huang S, Qin S et al (2012) Progesterone for acute traumatic brain injury. Cochrane Database Syst Rev 10:CD008409
Wright DW, Kellermann AL, Hertzberg VS et al (2007) Protect: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med 49:391–402, 402.e391–392
Judd HL, Meldrum DR, Deftos LJ et al (1983) Estrogen replacement therapy: indications and complications. Ann Intern Med 98:195–205
Raafat AM, Hofseth LJ, Haslam SZ (2001) Proliferative effects of combination estrogen and progesterone replacement therapy on the normal postmenopausal mammary gland in a murine model. Am J Obstet Gynecol 184:340–349
Hwang SL, Shih PH, Yen GC (2012) Neuroprotective effects of citrus flavonoids. J Agric Food Chem 60:877–885
Mazur W (1998) Phytoestrogen content in foods. Baillieres Clin Endocrinol Metab 12:729–742
Rong W, Wang J, Liu X et al (2012) Naringin treatment improves functional recovery by increasing BDNF and VEGF expression, inhibiting neuronal apoptosis after spinal cord injury. Neurochem Res 37:1615–1623
Kumar A, Prakash A, Dogra S (2010) Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by d-galactose in mice. Food Chem Toxicol 48:626–632
Gaur V, Aggarwal A, Kumar A (2009) Protective effect of naringin against ischemic reperfusion cerebral injury: possible neurobehavioral, biochemical and cellular alterations in rat brain. Eur J Pharmacol 616:147–154
Golechha M, Chaudhry U, Bhatia J et al (2011) Naringin protects against kainic acid-induced status epilepticus in rats: evidence for an antioxidant, anti-inflammatory and neuroprotective intervention. Biol Pharm Bull 34:360–365
Dajas F, Rivera-Megret F, Blasina F et al (2003) Neuroprotection by flavonoids. Braz J Med Biol Res 36:1613–1620
Menze ET, Tadros MG, Abdel-Tawab AM et al (2012) Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats. Neurotoxicology 33:1265–1275
Nones J, Spohr TCE, Gomes FC (2011) Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochem Res 36:1776–1784
Heo HJ, Kim DO, Shin SC et al (2004) Effect of antioxidant flavanone, naringenin, from citrus junoson neuroprotection. J Agric Food Chem 52:1520–1525
Youdim KA, Qaiser MZ, Begley DJ et al (2004) Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic Biol Med 36:592–604
Benavente-Garcia O, Castillo J (2008) Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem 56:6185–6205
Youdim KA, Spencer JP, Schroeter H et al (2002) Dietary flavonoids as potential neuroprotectants. Biol Chem 383:503–519
Liu Y, Su WW, Wang S et al (2012) Naringin inhibits chemokine production in an LPS-induced RAW 264.7 macrophage cell line. Mol Med Rep 6:1343–1350
Gopinath K, Sudhandiran G (2012) Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 227:134–143
Chanet A, Milenkovic D, Deval C et al (2012) Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J Nutr Biochem 23:469–477
Jagetia A, Jagetia GC, Jha S (2007) Naringin, a grapefruit flavanone, protects v79 cells against the bleomycin-induced genotoxicity and decline in survival. J Appl Toxicol 27:122–132
Prakash A, Shur B, Kumar A (2013) Naringin protects memory impairment and mitochondrial oxidative damage against aluminum-induced neurotoxicity in rats. Int J Neurosci 123:636–645
Fuhr U, Kummert AL (1995) The fate of naringin in humans: a key to grapefruit juice-drug interactions? Clin Pharmacol Ther 58:365–373
Zbarsky V, Datla KP, Parkar S et al (2005) Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res 39:1119–1125
Feeney DM, Boyeson MG, Linn RT et al (1981) Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res 211:67–77
Schabitz WR, Berger C, Kollmar R et al (2004) Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke 35:992–997
Hall ED, Vaishnav RA, Mustafa AG (2010) Antioxidant therapies for traumatic brain injury. Neurotherapeutics 7:51–61
Bains M, Hall ED (2012) Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta 1822:675–684
Ercan M, Inci S, Kilinc K et al (2001) Nimodipine attenuates lipid peroxidation during the acute phase of head trauma in rats. Neurosurg Rev 24:127–130
Kalayci M, Unal MM, Gul S et al (2011) Effect of coenzyme q10 on ischemia and neuronal damage in an experimental traumatic brain-injury model in rats. BMC Neurosci 12:75
Urso ML, Clarkson PM (2003) Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189:41–54
Cherian L, Hlatky R, Robertson CS (2004) Nitric oxide in traumatic brain injury. Brain Pathol 14:195–201
Clark RS, Kochanek PM, Schwarz MA et al (1996) Inducible nitric oxide synthase expression in cerebrovascular smooth muscle and neutrophils after traumatic brain injury in immature rats. Pediatr Res 39:784–790
Orihara Y, Ikematsu K, Tsuda R et al (2001) Induction of nitric oxide synthase by traumatic brain injury. Forensic Sci Int 123:142–149
Louin G, Marchand-Verrecchia C, Palmier B et al (2006) Selective inhibition of inducible nitric oxide synthase reduces neurological deficit but not cerebral edema following traumatic brain injury. Neuropharmacology 50:182–190
Gahm C, Holmin S, Wiklund PN et al (2006) Neuroprotection by selective inhibition of inducible nitric oxide synthase after experimental brain contusion. J Neurotrauma 23:1343–1354
Vavilala MS, Roberts JS, Moore AE et al (2001) The influence of inhaled nitric oxide on cerebral blood flow and metabolism in a child with traumatic brain injury. Anesth Analg 93:351–353, 353rd contents page
Goodman JC, Van M, Gopinath SP et al (2008) Pro-inflammatory and pro-apoptotic elements of the neuroinflammatory response are activated in traumatic brain injury. Acta Neurochir Suppl 102:437–439
White TE, Ford GD, Surles-Zeigler MC et al (2013) Gene expression patterns following unilateral traumatic brain injury reveals a local pro-inflammatory and remote anti-inflammatory response. BMC Genom 14:282
Das M, Mohapatra S, Mohapatra SS (2012) New perspectives on central and peripheral immune responses to acute traumatic brain injury. J Neuroinflammation 9:236
Allan SM, Tyrrell PJ, Rothwell NJ (2005) Interleukin-1 and neuronal injury. Nat Rev Immunol 5:629–640
Simi A, Tsakiri N, Wang P et al (2007) Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans 35:1122–1126
Utagawa A, Truettner JS, Dietrich WD et al (2008) Systemic inflammation exacerbates behavioral and histopathological consequences of isolated traumatic brain injury in rats. Exp Neurol 211:283–291
Tehranian R, Andell-Jonsson S, Beni SM et al (2002) Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma 19:939–951
Kinoshita K, Chatzipanteli K, Vitarbo E et al (2002) Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: Importance of injury severity and brain temperature. Neurosurgery 51:195–203 (discussion 203)
Tsai TH (2002) Determination of naringin in rat blood, brain, liver, and bile using microdialysis and its interaction with cyclosporin a, a p-glycoprotein modulator. J Agric Food Chem 50:6669–6674
Acknowledgments
This work was directed by Pro. Hong-yang Zhao from Union Hospital affiliated to Tongji Medical Collage of Huazhong University of Science and Technology, and helped by members from neuroscience research center of neurology, Tongji Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Qj., Wang, Ly., Wei, Zx. et al. Continual Naringin Treatment Benefits the Recovery of Traumatic Brain Injury in Rats Through Reducing Oxidative and Inflammatory Alterations. Neurochem Res 39, 1254–1262 (2014). https://doi.org/10.1007/s11064-014-1306-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1306-2