Abstract
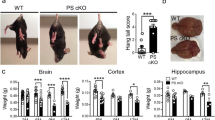
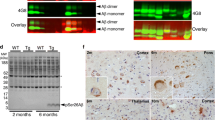
Mutations in presenilin 1 (PS-1) are associated with most early-onset familiar Alzheimer’s disease (AD). Previous studies have demonstrated that PS-1 mutations enhance the production of beta-amyloid (Aβ). In this study, we further examined the in vivo effects of PS-1 mutation on tau and synapse protein markers. The data showed that the phosphorylation of tau at Ser396, Ser404, Thr231 and Tau-1 (Ser198/199/202) epitopes was significantly increased in hippocampus of the aged (twenty-one and a half-month-old) transgenic mice expressing PS-1 (L235P) compared to that of the age-matched wild-type littermates (WTs). Concurrently, a significant decrease in the phosphorylation of glycogen synthase kinase (GSK)-3β at Ser9 was observed. The above changes were not observed in the young transgenic mice (6–8 months old). No significant changes in the levels of cyclin-dependent kinase (CDK)-5, its co-activator p35, and phosphorylation of protein phosphatase (PP)-2A catalytic subunit at Tyrosine 307 (Y307), a crucial site regulating the activity of PP-2A, were observed both in the young and aged transgenic mice compared to that of WTs. Furthermore, we also observed that the levels of presynaptic synaptophysin were significantly decreased but postsynaptic density protein (PSD)-95 were not significantly altered in hippocampus of the aged transgenic mice. No significant changes of synaptophysin or PSD-95 were observed in the brains of the young transgenic mice. Our data indicate that the L235P PS-1 mutation can induce Alzheimer-like tau hyperphosphorylation and synaptic protein loss, as well as increased production of Aβ.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Abbreviations
- AD:
-
Alzheimer’s disease
- PS-1:
-
Presenilin 1
- (GSK)-3β:
-
Glycogen synthase kinase-3β
- CDK-5:
-
Cyclin-dependent kinase-5
- PP-2A:
-
Protein phosphatase-2A
References
Selkoe DJ (1994) Alzheimer’s disease: a central role for amyloid. J Neuropathol Exp Neurol 53:438–447
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261:6084–6089
Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, Michon A, Martin C, Charbonnier F, Raux G, Camuzat A, Penet C, Mesnage V, Martinez M, Clerget-Darpoux F, Brice A, Frebourg T (1999) Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet 65:664–670
Cruts M, van Duijn CM, Backhovens H, Van den Broeck M, Wehnert A, Serneels S, Sherrington R, Hutton M, Hardy J, St George-Hyslop PH, Hofman A, Van Broeckhoven C (1998) Estimation of the genetic contribution of presenilin-1 and -2 mutations in a population-based study of presenile Alzheimer disease. Hum Mol Genet 7:43–51
Sherrington R, Froelich S, Sorbi S, Campion D, Chi H, Rogaeva EA, Levesque G, Rogaev EI, Lin C, Liang Y, Ikeda M, Mar L, Brice A, Agid Y, Percy ME, Clerget-Darpoux F, Piacentini S, Marcon G, Nacmias B, Amaducci L, Frebourg T, Lannfelt L, Rommens JM, St George-Hyslop PH (1996) Alzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum Mol Genet 5:985–988
Tanzi RE, Vaula G, Romano DM, Mortilla M, Huang TL, Tupler RG, Wasco W, Hyman BT, Haines JL, Jenkins BJ et al (1992) Assessment of amyloid beta-protein precursor gene mutations in a large set of familial and sporadic Alzheimer disease cases. Am J Hum Genet 51:273–282
Lleó A, Blesa R, Queralt R, Ezquerra M, Molinuevo JL, Peña-Casanova J, Rojo A, Oliva R (2002) Frequency of mutations in the presenilin and amyloid precursor protein genes in early-onset Alzheimer disease in Spain. Arch Neurol 59:1759–1763
Janssen JC, Beck JA, Campbell TA, Dickinson A, Fox NC, Harvey RJ, Houlden H, Rossor MN, Collinge J (2003) Early onset familial Alzheimer’s disease: mutation frequency in 31 families. Neurology 60:235–239
Gómez-Isla T, Growdon WB, McNamara MJ, Nochlin D, Bird TD, Arango JC, Lopera F, Kosik KS, Lantos PL, Cairns NJ, Hyman BT (1999) The impact of different presenilin 1 andpresenilin 2 mutations on amyloid deposition, neurofibrillary changes and neuronal loss in the familial Alzheimer’s disease brain: evidence for other phenotype-modifying factors. Brain 122:1709–1719
Xia W (2000) Role of presenilin in gamma-secretase cleavage of amyloid precursor protein. Exp Gerontol 35:453–460
Chui DH, Tanahashi H, Ozawa K, Ikeda S, Checler F, Ueda O, Suzuki H, Araki W, Inoue H, Shirotani K, Takahashi K, Gallyas F, Tabira T (1999) Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat Med 5:560–564
Yang X, Yang Y, Li G, Wang J, Yang ES (2008) Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci 34:165–171
Johnson GV, Stoothoff WH (2004) Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci 117:5721–5729
Wang JZ, Grundke-Iqbal I, Iqbal K (2007) Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci 25:59–68
Takashima A (2006) GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 9:309–317
Gozes I (2002) Tau as a drug target in Alzheimer’s disease. J Mol Neurosci 19:337–338
Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF (1999) Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 58:1010–1019
Mazanetz MP, Fischer PM (2007) Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov 6:464–479
Lau LF, Ahlijanian MK (2003) Role of cdk5 in the pathogenesis of Alzheimer’s disease. Neurosignals 12:209–214
Monaco EA (2004) Recent evidence regarding a role for Cdk5 dysregulation in Alzheimer’s disease. Curr Alzheimer Res 1:33–38
Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH (2001) p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci 21:6758–6771
Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA 97:2910–2915
Goedert M, Jakes R, Qi Z, Wang JH, Cohen P (1995) Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J Neurochem 65:2804–2807
Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K (1993) Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 61:921–927
Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2005) Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 22:1942–1950
Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K (1995) Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem 270:4854–4860
Chen J, Martin BL, Brautigan DL (1992) Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science 257:1261–1264
Blennow K, Bogdanovic N, Alafuzoff I, Ekman R, Davidsson P (1996) Synaptic pathology in Alzheimer’s disease: relation to severity of dementia, but not to senile plaques, neurofibrillary tangles, or the ApoE4 allele. J Neural Transm 103:603–618
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27:457–464
Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384
Leuba G, Walzer C, Vernay A, Carnal B, Kraftsik R, Piotton F, Marin P, Bouras C, Savioz A (2008) Postsynaptic density protein PSD-95 expression in Alzheimer’s disease and okadaic acid induced neuritic retraction. Neurobiol Dis 30:408–419
Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ (2006) Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging 27:797–803
Li G, Zou L, Jack CR Jr, Yang Y, Yang ES (2007) Neuroprotective effect of Coenzyme Q10 on ischemic hemisphere in aged mice with mutations in the amyloid precursor protein. Neurobiol Aging 28:877–882
Li G, Jack CR, Yang XF, Yang ES (2008) Diet supplement CoQ10 delays brain atrophy in aged transgenic mice with mutations in the amyloid precursor protein: an in vivo volume MRI study. Biofactors 32:169–178
Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA (2005) Tau phosphorylation in Alzheimer’s disease: pathogen or protector? Trends Mol Med 11:164–169
Gong CX, Iqbal K (2008) Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem 15:2321–2328
Strazielle C, Jazi R, Verdier Y, Qian S, Lalonde R (2009) Regional brain metabolism with cytochrome c oxidase histochemistry in a PS1/A246E mouse model of autosomal dominant Alzheimer’s disease: correlations with behavior and oxidative stress. Neurochem Int 55:806–814
Huang XG, Yee BK, Nag S, Chan ST, Tang F (2003) Behavioral and neurochemical characterization of transgenic mice carrying the human presenilin-1 gene with or without the leucine-to-proline mutation at codon 235. Exp Neurol 183:673–681
Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK (2004) PS1 activates PI3 K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J 23:2586–2596
Heinonen O, Soininen H, Sorvari H, Kosunen O, Paljärvi L, Koivisto E, Riekkinen PJ Sr (1995) Loss of synaptophysin-like immunoreactivity in the hippocampal formation is an early phenomenon in Alzheimer’s disease. Neuroscience 64:375–384
Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW Jr, Morris JC (2001) Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 56:127–129
Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM (2001) Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging 22:993–1005
Rutten BP, Van der Kolk NM, Schafer S, van Zandvoort MA, Bayer TA, Steinbusch HW, Schmitz C (2005) Age-related loss of synaptophysin immunoreactive presynaptic boutons within the hippocampus of APP751SL, PS1M146L, and APP751SL/PS1M146L transgenic mice. Am J Pathol 167:161–173
Pigino G, Pelsman A, Mori H, Busciglio J (2001) Presenilin-1 mutations reduce cytoskeletal association, deregulate neurite growth, and potentiate neuronal dystrophy and tau phosphorylation. J Neurosci 21:834–842
Terwel D, Dewachter I, Van Leuven F (2002) Axonal transport, tau protein, and neurodegeneration in Alzheimer’s disease. Neuromolecular Med 2:151–165
Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li WP, Mobley WC, Chu S (2007) One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci USA 104:13666–13671
Schindowski K, Belarbi K, Buée L (2008) Neurotrophic factors in Alzheimer’s disease: role of axonal transport. Genes Brain Behav 7:43–56
Gylys KH, Fein JA, Yang F, Wiley DJ, Miller CA, Cole GM (2004) Synaptic changes in Alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol 165:1809–1817
Leuba G, Savioz A, Vernay A, Carnal B, Kraftsik R, Tardif E, Riederer I, Riederer BM (2008) Differential changes in synaptic proteins in the Alzheimer frontal cortex with marked increase in PSD-95 postsynaptic protein. J Alzheimers Dis 15:139–151
Acknowledgments
This study was supported by the grant from National Natural Science Foundation of China (NSFC, 30700277).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xifei Yang and Ying Yang equally contributed to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2011_575_MOESM1_ESM.pdf
Supplementary Fig. 1. Phosphorylation of tau was not significantly altered in hippocampus of the young transgenic mice. The phosphorylation level of tau was normalized against total tau probed by Tau-5. Phosphorylation of tau at Ser396, Ser404, Thr231 and Tau-1 (Ser198/199/202) Ser 262 epitopes in hippocampus of the young transgenic mice was not significantly altered. Quantitative analysis showed the relative intensities of tau at above sites. (n=4). (PDF 60 kb)
11064_2011_575_MOESM2_ESM.pdf
Supplementary Fig. 2. The levels of p-GSK-3β in hippocampus of the young transgenic mice were not altered. The levels of p-GSK-3β/GSK-3β in hippocampus of the young transgenic mice were determined by Western blot analysis. Quantitative analysis showed the relative intensity of p-GSK-3β/total GSK-3β. (n=4). (PDF 39 kb)
11064_2011_575_MOESM3_ESM.pdf
Supplementary Fig. 3. The levels of synaptophysin and PSD-95 in hippocampus of the young transgenic mice were not altered. The levels of synaptophysin and PSD-95 in hippocampus of the young transgenic mice were determined by Western blot analysis. The levels of synaptophysin and PSD-95 were normalized against β-actin. Quantitative analysis showed the relative intensities of synaptophysin and PSD-95. (n=4). (PDF 47 kb)
Rights and permissions
About this article
Cite this article
Yang, X., Yang, Y., Liu, J. et al. Increased Phosphorylation of Tau and Synaptic Protein Loss in the Aged Transgenic Mice Expressing Familiar Alzheimer’s Disease-Linked Presenilin 1 Mutation. Neurochem Res 37, 15–22 (2012). https://doi.org/10.1007/s11064-011-0575-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0575-2