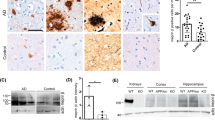
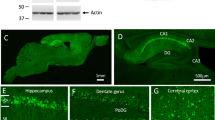
Abstract
Alzheimer’s disease, responsible for the vast majority of dementia cases in the elderly population, is caused by accumulation of toxic levels of amyloid β peptide (Aβ) in the brain. Neprilysin is a major enzyme responsible for the degradation of Aβ in vivo. We have previously shown that elevation of neprilysin levels in the brain delays the deposition of Aβ -plaques in a mouse model of amyloidosis and that lack of neprilysin leads to increased Aβ generation and to signs of incipient neurodegeneration in mouse brains. This study was designed to test whether low brain levels of neprilysin affect the amyloid pathology or perturb the learning and memory performance of mice. Double-mutated mice carrying a targeted depletion of one allele of Mme, the gene encoding neprilysin, and over-expressing human amyloid precursor protein (APP), exhibited a reinforced amyloid pathology in comparison with their APP transgenic littermates. Moreover, in contrast to their parental lines, these mice were impaired in the Morris water maze learning and memory paradigm and showed facilitated extinction in the conditioned taste aversion test. These data suggest that even a partial neprilysin deficiency, as is found during aging, exacerbates amyloid pathology and may impair cognitive functions.





Similar content being viewed by others
References
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. doi:10.1126/science.1072994
Mohajeri MH, Saini K, Li H et al (2003) Intact spatial memory in mice with seizure-induced partial loss of hippocampal pyramidal neurons. Neurobiol Dis 12:174–181. doi:10.1016/S0969-9961(02)00031-1
Reed JM, Squire LR (1997) Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci 111:667–675. doi:10.1037/0735-7044.111.4.667
Rempel-Clower NL, Zola SM, Squire LR, Amaral DG (1996) Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci 16:5233–5255
Tonegawa S, Tsien JZ, McHugh TJ, Huerta P, Blum KI, Wilson MA (1996) Hippocampal CA1-region-restricted knockout of NMDAR1 gene disrupts synaptic plasticity, place fields, and spatial learning. Cold Spring Harb Symp Quant Biol 61:225–238
Iwata N, Higuchi M, Saido TC (2005) Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacol Ther 108:129–148. doi:10.1016/j.pharmthera.2005.03.010
Saido TC (1998) Alzheimer’s disease as proteolytic disorders: anabolism and catabolism of beta-amyloid. Neurobiol Aging 19:S69–S75. doi:10.1016/S0197-4580(98)00033-5
Iwata N, Tsubuki S, Takaki Y et al (2001) Metabolic regulation of brain Abeta by neprilysin. Science 292:1550–1552. doi:10.1126/science.1059946
Iwata N, Tsubuki S, Takaki Y et al (2000) Identification of the major Abeta1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med 6:143–150. doi:10.1038/77399
Yasojima K, Akiyama H, McGeer EG, McGeer PL (2001) Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide. Neurosci Lett 297:97–100. doi:10.1016/S0304-3940(00)01675-X
Yasojima K, McGeer EG, McGeer PL (2001) Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res 919:115–121. doi:10.1016/S0006-8993(01)03008-6
Lazarov O, Robinson J, Tang YP et al (2005) Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 120:701–713. doi:10.1016/j.cell.2005.01.015
Huang SM, Mouri A, Kokubo H et al (2006) Neprilysin-sensitive synapse-associated Amyloid-beta peptide oligomers impair neuronal plasticity and cognitive function. J Biol Chem 281:17941–17951. doi:10.1074/jbc.M601372200
Madani R, Poirier R, Wolfer DP et al (2006) Lack of neprilysin suffices to generate murine amyloid-like deposits in the brain and behavioral deficit in vivo. J Neurosci Res 84:1871–1878. doi:10.1002/jnr.21074
Mohajeri MH, Wollmer MA, Nitsch RM (2002) Abeta 42-induced increase in neprilysin is associated with prevention of amyloid plaque formation in vivo. J Biol Chem 277:35460–35465. doi:10.1074/jbc.M202899200
Lu B, Gerard NP, Kolakowski LF Jr et al (1995) Neutral endopeptidase modulation of septic shock. J Exp Med 181:2271–2275. doi:10.1084/jem.181.6.2271
Mucke L, Masliah E, Yu GQ et al (2000) High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20:4050–4058
Poirier R, WD P, Welzl H et al (2006) Neuronal neprilysin overexpression is associated with attenuation of Aβ -related spatial memory deficit. Neurobiol Dis 24:475–483. doi:10.1016/j.nbd.2006.08.003
Mohajeri MH, Saini K, Schultz JG, Wollmer MA, Hock C, Nitsch RM (2002) Passive immunization against beta-amyloid peptide protects central nervous system (CNS) neurons from increased vulnerability associated with an Alzheimer’s disease-causing mutation. J Biol Chem 277:33012–33017. doi:10.1074/jbc.M203193200
Shirotani K, Tsubuki S, Iwata N et al (2001) Neprilysin degrades both amyloid beta peptides 1–40 and 1–42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J Biol Chem 276:21895–21901. doi:10.1074/jbc.M008511200
Wolfer DP, Madani R, Valenti P, Lipp HP (2001) Extended analysis of path data from mutant mice using the public domain software Wintrack. Physiol Behav 73:745–753. doi:10.1016/S0031-9384(01)00531-5
Janus C, Welzl H, Hanna A et al (2004) Impaired conditioned taste aversion learning in APP transgenic mice. Neurobiol Aging 25:1213–1219. doi:10.1016/j.neurobiolaging.2003.11.007
Welzl H, D’Adamo P, Lipp HP (2001) Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res 125:205–213. doi:10.1016/S0166-4328(01)00302-3
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791. doi:10.1126/science.1074069
Brendza RP, Bacskai BJ, Cirrito JR et al (2005) Anti- Aβ antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J Clin Invest 115:428–433
Lombardo JA, Stern EA, McLellan ME et al (2003) Amyloid-beta antibody treatment leads to rapid normalization of plaque-induced neuritic alterations. J Neurosci 23:10879–10883
Tsai J, Grutzendler J, Duff K, Gan WB (2004) Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci 7:1181–1183. doi:10.1038/nn1335
Saido TC, Iwata N (2006) Metabolism of amyloid beta peptide and pathogenesis of Alzheimer’s disease. Towards presymptomatic diagnosis, prevention and therapy. Neurosci Res 54:235–253. doi:10.1016/j.neures.2005.12.015
Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM (2005) Age- and region-dependent alterations in Abeta-degrading enzymes: implications for Abeta-induced disorders. Neurobiol Aging 26:645–654. doi:10.1016/j.neurobiolaging.2004.06.013
Iwata N, Takaki Y, Fukami S, Tsubuki S, Saido TC (2002) Region-specific reduction of A beta-degrading endopeptidase, neprilysin, in mouse hippocampus upon aging. J Neurosci Res 70:493–500. doi:10.1002/jnr.10390
Wang DS, Iwata N, Hama E, Saido TC, Dickson DW (2003) Oxidized neprilysin in aging and Alzheimer’s disease brains. Biochem Biophys Res Commun 310:236–241. doi:10.1016/j.bbrc.2003.09.003
Fukuchi K, Sopher B, Furlong CE, Smith AC, Dang N, Martin GM (1993) Selective neurotoxicity of COOH-terminal fragments of the beta-amyloid precursor protein. Neurosci Lett 154:145–148. doi:10.1016/0304-3940(93)90192-N
Sopher BL, Fukuchi K, Smith AC, Leppig KA, Furlong CE, Martin GM (1994) Cytotoxicity mediated by conditional expression of a carboxyl-terminal derivative of the beta-amyloid precursor protein. Brain Res Mol Brain Res 26:207–217. doi:10.1016/0169-328X(94)90092-2
Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL (1989) Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science 245:417–420. doi:10.1126/science.2474201
Cullen WK, Suh YH, Anwyl R, Rowan MJ (1997) Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport 8:3213–3217
Nalbantoglu J, Tirado-Santiago G, Lahsaini A et al (1997) Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature 387:500–505. doi:10.1038/387500a0
Berger-Sweeney J, McPhie DL, Arters JA, Greenan J, Oster-Granite ML, Neve RL (1999) Impairments in learning and memory accompanied by neurodegeneration in mice transgenic for the carboxyl-terminus of the amyloid precursor protein. Brain Res Mol Brain Res 66:150–162. doi:10.1016/S0169-328X(99)00014-5
Oster-Granite ML, McPhie DL, Greenan J, Neve RL (1996) Age-dependent neuronal and synaptic degeneration in mice transgenic for the C terminus of the amyloid precursor protein. J Neurosci 16:6732–6741
Ishida A, Furukawa K, Keller JN, Mattson MP (1997) Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport 8:2133–2137. doi:10.1097/00001756-199707070-00009
Mattson MP (1997) Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev 77:1081–1132
Pardossi-Piquard R, Petit A, Kawarai T et al (2005) Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP. Neuron 46:541–554. doi:10.1016/j.neuron.2005.04.008
Dawson GR, Seabrook GR, Zheng H et al (1999) Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience 90:1–13. doi:10.1016/S0306-4522(98)00410-2
Seabrook GR, Smith DW, Bowery BJ et al (1999) Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology 38:349–359. doi:10.1016/S0028-3908(98)00204-4
Ring S, Weyer SW, Kilian SB et al (2007) The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci 27:7817–7982. doi:10.1523/JNEUROSCI.1026-07.2007
Moechars D, Dewachter I, Lorent K et al (1999) Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem 274:6483–6492. doi:10.1074/jbc.274.10.6483
Howell S, Nalbantoglu J, Crine P (1995) Neutral endopeptidase can hydrolyze beta-amyloid (1–40) but shows no effect on beta-amyloid precursor protein metabolism. Peptides 16:647–652. doi:10.1016/0196-9781(95)00021-B
Leissring MA, Farris W, Chang AY et al (2003) Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40:1087–1093. doi:10.1016/S0896-6273(03)00787-6
Hardy J (1997) Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci 20:154–159. doi:10.1016/S0166-2236(96)01030-2
Selkoe DJ (1998) The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol 8:447–453. doi:10.1016/S0962-8924(98)01363-4
Hama E, Shirotani K, Masumoto H, Sekine-Aizawa Y, Aizawa H, Saido TC (2001) Clearance of extracellular and cell-associated amyloid beta peptide through viral expression of neprilysin in primary neurons. J Biochem 130:721–726
Crameri A, Biondi E, Kuehnle K et al (2006) The role of seladin-1/DHCR24 in cholesterol biosynthesis, APP processing and Abeta generation in vivo. EMBO J 25:432–443. doi:10.1038/sj.emboj.7600938
Refolo LM, Pappolla MA, LaFrancois J et al (2001) A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 8:890–899. doi:10.1006/nbdi.2001.0422
Acknowledgments
We thank Drs. L. Mucke (Gladstone Institute, UCSF, CA) and B. Lu (Pulmonary Division, Children’s Hospital, Boston, MA) for providing the APPtg and neprilysin deficient mice and I. Drescher, O. Litvin, J. Tracy, H. Müller and H. Li for technical help. We are also thankful to H.-P. Lipp for helpful discussions and A. Mechan for critically reading this manuscript. This work was supported by grants from the University of Zurich, NCCR Neural Plasticity and Repair, the Desirée and Niels Yde, Mobiliar and EMDO foundations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue to Honor Dr. Akitane Mori.
Rights and permissions
About this article
Cite this article
Mohajeri, M.H., Wolfer, D.P. Neprilysin Deficiency-Dependent Impairment of Cognitive Functions in a Mouse Model of Amyloidosis. Neurochem Res 34, 717–726 (2009). https://doi.org/10.1007/s11064-009-9919-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-9919-6