Abstract
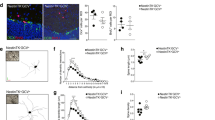
Lesions of the nigrostriatal pathway are known to induce a compensatory up-regulation of various neurotrophic factors. In this study we examined protein content of basic fibroblast growth factor (FGF-2) in tissue samples taken from the ventral midbrain and striatum at two different time points following a neurotoxic lesion of the nigrostriatal pathway in two different rat strains, the outbred Sprague–Dawley (SD) and inbred F344 × Brown Norway F1 hybrid (F344BNF1). Despite both rat strains having comparable lesions of the nigrostriatal pathway, we observed a difference in the temporal up-regulation of FGF-2 in ventral midbrain samples taken from the side ipsilateral to the lesion. Basic FGF was significantly up-regulated in ventral midbrain in SD rats 1 week post-lesion while we did not observe an up-regulation of FGF-2 in the lesioned ventral midbrain of F344BNF1 at this same time point. However, both strains showed a significant up-regulation of FGF-2 in the lesioned ventral midbrain 3 weeks post-lesion. Sprague–Dawley rats also appeared to be more sensitive to the lesion in terms of up-regulating FGF-2 expression. The differences reported here suggest currently unknown genetic differences between these two strains may be important factors for regulating the compensatory release of neurotrophic factors, such as FGF-2, in response to a neurotoxic lesion of the nigrostriatal pathway.





Similar content being viewed by others
References
Eckenstein FP (1994) Fibroblast growth factors in the nervous system. J Neurobiol 25:1467–1480
Ornitz DM, Itoh N (2001) Fibroblast growth factors. Genome Biol 2:1–10
Dono R (2003) Fibroblast growth factors as regulators of central nervous system development and function. Am J Physiol 284:R867–R881
Grothe C, Timmer M (2007) The physiological and pharmacological role of basic fibroblast growth factor in the dopaminergic nigrostriatal system. Brain Res Rev 54:80–91
Bean AJ, Elde R, Cao YH et al (1991) Expression of acidic and basic fibroblast growth factors in the substantia nigra of rat, monkey, and human. Proc Natl Acad Sci USA 88:10237–10241
Tooyama I, Walker D, Yamada T et al (1992) High molecular weight basic fibroblast growth factor-like protein is localized to a subpopulation of mesencephalic dopaminergic neurons in the rat brain. Brain Res 593:274–280
Fawcett JW, Barker RA, Dunnett SB (1995) Dopaminergic neuronal survival and the effects of bFGF in explant, three dimensional and monolayer cultures of embryonic rat ventral mesencephalon, Exp. Brain Res 106:275–282
Ferrari G, Minozzi MC, Toffano G et al (1989) Basic fibroblast growth factor promotes the survival and development of mesencephalic neurons in culture. Dev Biol 133:140–147
Knüsel B, Michel PP, Schwaber JS et al (1990) Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J Neurosci 10:558–570
Mayer E, Dunnett SB, Pellitteri R et al (1993) Basic fibroblast growth factor promotes the survival of embryonic ventral mesencaphalic dopaminergic neurons-I. Effects in vitro. Neuroscience 56:379–388
Engele J, Schubert D, Bohn MC (1991) Conditioned media derived from glial cell lines promote survival and differentiation of dopaminergic neurons in vitro: role of mesencephalic glia. J Neurosci Res 30:359–371
Zeng B-Y, Jenner P, Marsden CD (1996) Altered motor function and graft survival produced by basic fibroblast growth factor in rats with 6-OHDA lesions and fetal ventral mesencephalic grafts are associated with glial proliferation. Exp Neurol 139:214–226
Yurek DM, Fletcher-Turner A (2000) Lesion-induced increase of BDNF is greater in the striatum of young versus old rat brain. Exp Neurol 161:392–396
Yurek DM, Fletcher-Turner A (2001) Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res 891:228–235
Zhou J, Pliego-Rivero B, Bradford HF et al (1996) The BDNF content of postnatal and adult rat brain: the effects of 6-hydroxydopamine lesions in adult brain. Brain Res Dev Brain Res 97:297–303
Funa K, Yamada N, Brodin G et al (1996) Enhanced synthesis of platelet-derived growth factor following injury induced by 6-hydroxydopamine in rat brain. Neuroscience 74:825–833
Collier TJ, Dung Ling Z, Carvey PM et al (2005) Striatal trophic factor activity in aging monkeys with unilateral MPTP-induced parkinsonism. Exp Neurol 191(Suppl 1):S60–S67
Claus P, Werner S, Timmer M et al (2004) Expression of the fibroblast growth factor-2 isoforms and the FGF receptor 1–4 transcripts in the rat model system of Parkinson’s disease. Neurosci Lett 360:117–120
Nakajima K, Hida H, Shimano Y et al (2001) GDNF is a major component of trophic activity in DA-depleted striatum for survival and neurite extension of DAergic neurons. Brain Res 916:76–84
Chadi G, Cao Y, Pettersson RF et al (1994) Temporal and spatial increase of astroglial basic fibroblast growth factor synthesis after 6-hydroxydopamine-induced degeneration of the nigrostriatal dopamine neurons. Neuroscience 61:891–910
Gomide V, Chadi G (2005) Glial bFGF and S100 immunoreactivities increase in ascending dopamine pathways following striatal 6-OHDA-induced partial lesion of the nigrostriatal system: a sterological analysis. Int J Neurosci 115:537–555
Leonard S, Luthman D, Logel J et al (1993) Acidic and basic fibroblast growth factor mRNAs are increased in striatum following MPTP-induced dopamine neurofiber lesion: assay by quantitative PCR, Mol. Brain Res 18:275–284
Rufer M, Wirth SB, Hofer A et al (1996) Regulation of connexin-43, GFAP, and FGF-2 is not accompanied by changes in astroglial coupling in MPTP-lesioned, FGF-2-treated parkinsonian mice. J Neurosci Res 46:606–617
Nakagawa T, Schwartz JP (2004) Gene expression profiles of reactive astrocytes in dopamine-depleted striatum. Brain Pathol 14:275–280
Tooyama I, Kawamata T, Walker D et al (1993) Loss of basic fibroblast growth factor in substantia nigra neurons in Parkinson’s disease. Neurology 43:372–376
Tooyama I, McGeer EG, Kawamata T et al (1994) Retention of basic fibroblast growth factor immunoreactivity in dopaminergic neurons of the substantia nigra during normal aging in humans contrasts with loss in Parkinson’s disease. Brain Res 656:165–168
Katoh-Semba R, Semba R, Takeuchi IK et al (1998) Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: a comparison to those of nerve growth factor and neurotrophin-3. Neurosci Res 31:227–234
Nishizuka M, Katoh-Semba R, Eto K et al (1991) Age- and sex-related differences in the nerve growth factor distribution in the rat brain, Brain Res Bull 27:685–688
Dhabhar FS, McEwen BS, Spencer RL (1993) Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels—a comparison between Sprague–Dawley, Fischer 344 and Lewis rats. Brain Res 616:89–98
Gao G, Li Y, Fant J et al (2002) Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium–derived factor in brown norway and sprague dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes 51:1218–1225
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Academic Press, New York
Shimasaki S, Emoto N, Koba A et al (1988) Complementary DNA cloning and sequencing of rat ovarian basic fibroblast growth factor and tissue distribution study of its mRNA. Biochem Biophys Res Commun 157:256–263
Cass WA, Harned ME, Peters LE et al (2003) HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res 984:133–142
Jeon BS, Jackson-Lewis V, Burke RE (1995) 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration 4:131–137
Lipman RD, Chrisp CE, Hazzard DG et al (1996) Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci 51:B54–59
Barberi T, Klivenyi P, Calingasan NY et al (2003) Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol 21:1200–1207
Lee SH, Lumelsky N, Studer L et al (2000) Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol 18:675–979
Yurek DM, Fletcher-Turner A (2004) Comparison of embryonic stem cell-derived dopamine neuron grafts and fetal ventral mesencephalic tissue grafts: morphology and function. Cell Transplant 13:295–306
Acknowledgments
This research was supported by DOD grant DAMD 17-01-1-0786 (DMY) and NIH grants NS50311 (DMY) and AG17963 (WAC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yurek, D.M., Fletcher, A.M., Peters, L.E. et al. Strain Difference in the Up-Regulation of FGF-2 Protein Following a Neurotoxic Lesion of the Nigrostriatal Pathway. Neurochem Res 35, 531–539 (2010). https://doi.org/10.1007/s11064-009-0093-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-0093-7