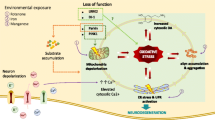
Abstract
Parkinson’s disease (PD) is the second most prevalent age-related neurodegenerative disease with physiological manifestations including tremors, bradykinesia, abnormal postural reflexes, rigidity and akinesia and pathological landmarks showing losses of dopaminergic neurons in the substantia nigra. Although the etiology of PD has been intensively pursued for several decades, biochemical mechanisms and genetic and epigenetic factors leading to initiation and progression of the disease remain elusive. Environmental toxins including (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) MPTP, paraquat and rotenone have been shown to increase the risk of PD in humans. Oxidative stress remains the leading theory for explaining progression of PD. Studies with cell and animal models reveal oxidative and inflammatory properties of these toxins and their ability to activate glial cells which subsequently destroy neighboring dopaminergic neurons. This review describes pathological effects of neurotoxins on cells and signaling pathways for production of reactive oxygen species (ROS) that underline the pathophysiology of PD.
Similar content being viewed by others
References
Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ (2006) Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res 3:327–337
Betarbet R, Sherer TB, Greenamyre JT (2005) Ubiquitin-proteasome system and Parkinson’s diseases. Exp Neurol 191:S17–S27
Mariani E, Polidori MC, Cherubini A, Mecocci P (2005) Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B: Analyt Technol Biomed Life Sci 827:65–75
Mattson MP (2006) Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal 8:1997–2006
Moreira PI, Siedlak SL, Aliev G, Zhu X, Cash AD, Smith MA, Perry G (2005) Oxidative stress mechanisms and potential therapeutics in Alzheimer disease. J Neural Transm 112:921–932
Cardoso SM, Moreira PI, Agostinho P, Pereira C, Oliveira CR (2005) Neurodegenerative pathways in Parkinson’s disease: therapeutic strategies. Curr Drug Targets CNS Neurolog Disord 4:405–419
Chun HS, Gibson GE, DeGiorgio LA, Zhang H, Kidd VJ, Son JH (2001) Dopaminergic cell death induced by MPP+, oxidant and specific neurotoxicants shares the common molecular mechanism. J Neurochem 76:1010–1021
Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318:215–224
Siderowf A, Stern M (2003) Update on Parkinson’s disease. Ann Intern Med 138:651–658
Gao HM, Liu B, Zhang W, Hong JS (2003) Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J 17:1954–1956
Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Harding M, Bellen H, Mardon G (2004) Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Dev Disease 131:2183–2194
Peng J, Stevenson FF, Doctrow SR, Andersen JK (2005) Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra. J Biol Chem 280:29194–29198
Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA (2000) The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci Res 20:9207–9214
Gelinas S, Martinoli M-G (2002) Neuroprotective effect of estradiol and phytoestrogens on MPP+-induced cytotoxicity in neuronal PC12 cells. J Neurosci Res 70:90–96
Uversky VN (2004) Neurotoxicant-induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissure Res 318:225–241
Tieu K, Ischiropoulos H, Przedborski S (2003) Nitric oxide and reactive oxygen species in Parkinson’s disease. IUBMB Life 55:329–335
Ahlskog JE (2005) Challenging conventional wisdom: the etiologic role of dopamine oxidative stress in Parkinson’s disease. Movement Disord 20:271–282
Beal MF (2004) Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr 36:381–386
Gandhi S, Wood NW (2005) Molecular pathogenesis of Parkinson’s disease. Human Mol Genet 14:2749–2755
Hald A, Lotharius J (2005) Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp Neurol 193:279–290
Greenamyre JT, Sherer TB, Betarbet R, Panov AV (2001) Complex I and Parkinson’s disease. IUBMB Life 52:135–141
Maguire-Zeiss KA, Short DW, Federoff HJ (2005) Synuclein, dopamine and oxidative stress: co-conspirators in Parkinson’s disease? Brain Research. Mol Brain Res 134:18–23
Firestone JA, Smith-Weller T, Franklin G, Swanson P, Lonstreth WT, Checkoway H (2005) Pesticides and risk of Parkinson disease. Arch Neurol 62:91–95
Semchuk KM, Love EJ, Lee RG (1993) Parkinson’s disease: a test of the multifactorial etiologic hypothesis. Neurology 43:1173–1180
Shimizu K, Matsubara K, Ohtaki K, Fujimaru S, Saito O, Shiono H (2003) Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res 976:243–252
Yang W-L, Sun AY (1998) Paraquat-induced free radical reaction in mouse brain microsomes. Neurochem Res 23:47–53
Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ (1999) Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res 823:1–10
Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC (1997) Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology 48:1583–1588
Bove J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2:484–494
Wersinger C, Sidhu A (2006) An inflammatory pathomechanism for Parkinson’s disease? Curr Med Chem 13:591–602
Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D (2005) Early environmental origins of neurodegenerative disease in later life. Environ Health Persp 113:1230–1233
Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS (2006) Pesticides and Parkinson’s disease-is there a link? Environ Health Persp 114:156–164
Schmidt WJ, Alam M (2006) Controversies on new animal models of Parkinson’s disease pro and con: the rotenone model of Parkinson’s disease (PD). J Neural Transm Suppl 273–276
Kotake Y, Ohta S (2003) MPP+ analogs acting on mitochondria and inducing neuro-degeneration. Curr Med Chem 10:2507–2516
Wesseling C, van Wendel de Joode B, Ruepert C, Leon C, Monge P, Hermosillo H, Partanen TJ (2001) Paraquat in developing countries. Int J Occup Environ Health 7:275–286
Yang W, Tiffany-Castiglioni E (2005) The bipyridyl herbicide paraquat produces oxidative stress-mediated toxicity in human neuroblastoma SH-SY5Y cells: relevance to the dopaminergic pathogenesis. J Toxicol Environ Health Part A 68:1939–1961
Shimizu K, Matsubara K, Ohtaki K, Shiono H (2003) Paraquat leads to dopaminergic neural vulnerability in organotypic midbrain culture. Neurosci Res 46:523–532
McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA (2002) Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 10:119–127
Yang W-L, Sun AY (1998) Paraquat-induced cell death in PC12 cells. Neurochem Res 23:1387–1394
Kang D, Miyako K, Kuribayashi F, Hasegawa E (1997) Changes in energy metabolism induced by 1-methyl-4-phenylpyridinium (MPP+)-related compounds in rat pheochromocytoma PC12 cells. Arch Biochem Biophys 337:75–80
McCormack A, Di Monte D (2003) Effect of l-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J Neurochem 85:82–86
Thiruchelvam M, Prokopenko O, Cory-Slechta D, Richfield E, Buckley B, Mirochnitchenko O (2005) Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. J Biol Chem 280:22530–22539
Bretaud S, Lee S, Guo S (2004) Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol Teratol 26:857–864
Block ML, Li G, Qin L, Wu X, Pei Z, Wang T, Wilson B, Yang J, Hong JS (2006) Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. FASEB J 20:251–258
Li G, Cui G, Tzeng NS, Wei SJ, Wang T, Block ML, Hong JS (2005) Femtomolar concentrations of dextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage. FASEB J 19:489–496
Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA (2005) Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. PNAS 102:9936–9941
Qin L, Liu Y, Qian X, Hong JS, Block ML (2005) Microglial NADPH oxidase mediates leucine enkephalin dopaminergic neuroprotection. Ann NY Acad Sci 1053:107–120
Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19:533–542
Arai H, Furuya T, Yasuda T, Miura M, Mizuno Y, Mochizuki H (2004) Neurotoxic effects of lipopolysaccharide on nigral dopaminergic neurons are mediated by microglial activation, interleukin-1beta, and expression of caspase-11 in mice. J Biol Chem 279:51647–51653
McGeer PL, Itagaki S, Akiyama H, McGeer EG (1988) Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol 24:574–576
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291
Mander PK, Jekabsone A, Brown GC (2006) Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol 176:1046–1052
Dringen R (2005) Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal 7:1223–1233
Delgado M, Ganea D (2003) Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson’s disease by blocking microglial activation. FASEB J 17:944–946
Gao HM, Hong JS, Zhang W, Liu B (2002) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790
Scheller C, Sopper S, Jenuwein M, Neuen-Jacob E, Tatschner T, Grunblatt E, ter Meulen V, Riederer P, Koutsilieri E (2005) Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J Neurochem 95:377–387
Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM (2004) Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther 311:1–7
Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S (2003) NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. PNAS 100:6145–6150
Barcia C, Bahillo AS, Fernandez-Villalba E, Bautista V, Poza MP, Fernandez-Barreiro A, Hirsch EC, Herrero M-T (2004) Evidence of the active microglia in substantia nigra pars compacta of Parkinsonian monkeys 1 year after MPTP exposure. GLIA 46:402–409
Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D (1999) Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol 46:598–605
Casarejos MJ, Menendez J, Solano RM, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA (2006) Susceptibility of rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J Neurochem 97:934–946
Croisier E, Moran LB, Dexter DT, Pearce RKB, Graeber MB (2005) Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation 2:14–22
Miller R, Sun G, Sun A (2007) Cytotoxicity of paraquat in microglial cells: involvement of the PKC delta- and ERK 1/2-dependent NADPH oxidase. Brain Res 1167:129–139
Sumimoto H, Ueno N, Yamasaki T, Taura M, Takeya R (2004) Molecular mechanism underlying activation of superoxide-producing NADPH oxidases: roles for their regulatory proteins. Jpn J Infect Dis 57:S24–S25
Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS (2004) NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem 279:1415–1421
Infanger DW, Sharma RV, Davisson RL (2006) NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8:1583–1596
Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA (2005) Redox cycling of the herbicide paraquat in microglial cultures Brain Res Mol Brain Res 134:52–56
Wu XF, Block ML, Zhang W, Qin L, Wilson B, Zhang WQ, Veronesi B, Hong JS (2005) The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal 7:654–661
Gao HM, Liu B, Hong JS (2003) Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopamingeric neurons. J Neurosci 23:6181–6187
Gao HM, Liu B, Zhang W, Hong JS (2003) Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. FASEB J 17:1957–1959
Gao H-M, Hong J-S, Zhang W, Liu B (2003) Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. J Neurosci 23:1228–1236
Wagey R, Hu J, Pelech SL, Raymond LA, Krieger C (2001) Modulation of NMDA-mediated excitotoxicity by protein kinase C. J Neurochem 78:715–726
Ran X, Miao H-H, Sheu F-S, Yan D (2003) Structural and dynamic characterization of a neuron-specific protein kinase C substrate, neurogranin. Biochemistry 42:5143–5150
Catarsi S, Drapeau P (1997) Requirement for tyrosine phosphatase during serotonergic neuromodulation by protein kinase C J Neurosci Res 17:5792–5797
Ahlemeyer B, Kolker S, Zhu Y, Hoffmann GF, Krieglstein J (2002) Increase in glutamate-induced neurotoxicity by activated astrocytes involves stimulation of protein kinase C. J Neurochem 82:504–515
Battaini F (2001) Protein kinase C isoforms as therapeutic targets in nervous system disease states. Pharmacol Res 44:353–361
Kozikowski AP, Nowak I, Petukhov PA, Etcheberrigaray R, Mohamed A, Tan M, Lewin N, Hennings H, Pearce LL, Blumber PM (2003) New amide-bearing benzolactam-based protein kinase C modulators induce enhanced secretion of the amyloid precursor protein metabolite sAPPα. J Med Chem 46:364–373
Gallagher HC, Murphy KJ, Foley AG, Regan CM (2001) Protein kinase C delta regulates neural cell adhesion molecule polysialylation state in the rat brain. J Neurochem 77:425–434
DeVries TA, Neville MC, Reyland ME (2002) Nuclear import of PKCδ is required for apoptosis: identification of a novel nuclear import sequence. EMBO J 21:6050–6060
Shibukawa Y, Takahashi M, Laffont I, Honke K, Taniguchi N (2003) Down-regulation of hydrogen peroxide-induced PKCd activation in N-acetylglucosaminyltransferase III-transfected HeLaS3 cells. J Biol Chem 278:3197–3203
Cataisson C, Joseloff E, Murillas R, Wang A, Atwell C, Torgerson S, Gerdes M, Subleski J, Gao J-L, Murphy PM, Wiltrout RH, Vinson C, Yuspa SH (2003) Activation of cutaneous protein kinase Ca induces keratinocyte apoptosis and intraepidermal signaling pathways. J Immunol 171:2603–2713
Leverrier S, Vallentin A, Joubert D (2002) Positive feedback of protein kinase C proteolytic activation during apoptosis. Biochem J 368:905–913
Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG (2002) Caspase-3-dependent proteolytic cleavage of protein kinase Cδ is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci Res 22:1738–1751
Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK (2004) Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173:5730–5738
He R, Nanamor M, Sang H, Yin H, Dinauer MC, Ye RD (2004) Reconstitution of chemotactic peptide-induced nicotinamide adenine dinucleotide phosphate (reduced)oxidase activation in transgenic COS-phox cells. J Immunol 173:7462–7470
Shao MX, Nadel JA (2005) Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. PNAS 102:767–772
Talior I, Tennebaum T, Kuroki T, Eldar-Finkelman H (2005) PKC-delta-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: role for NADPH oxidase. Am J Physiol-Endocrinol Metab 288:E405–E411
Waki K, Inanami O, Yamamori T, Nagahata H, Kuwabara M (2006) Involvement of protein kinase Cdelta in the activation of NADPH oxidase and the phagocytosis of neutrophils. Free Radic Res 40:359–367
Zhang X, Dong F, Ren J, Driscoll MJ, Culver B (2005) High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol 191:318–325
Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M (2005) Leukotrienes enhance the bactericidal activity of alveolare macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 106:1067–1075
Zhao X, Xu B, Bhattacharjee A, Oldfield CM, Wientjes FB, Feldman GM, Cathcart MK (2005) Protein kinase Cdelta regulates p67phox phosphorylation in human monocytes. J Leukocyte Biol 77:414–420
Nishimoto S, Nishida E (2006) MAPK signalling: ERK5 versus ERK1/2. EMBO Rep 7:782–786
Bardwell L (2006) Mechanisms of MAPK signalling specificity. Biochem Soc Trans 34:837–841
Cuschieri J, Maier RV (2005) Mitogen-activated protein kinase (MAPK). Crit Care Med 33:S417–S419
Kaminska B (2005) MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754:253–262
Uhlik MT, Abell AN, Cuevas BD, Nakamura K, Johnson GL (2004) Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem Cell Biol 82:658–663
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68:320–344
Murphy LO, Blenis J (2006) MAPK signal specificity: the right place at the right time. Trend Biochem Sci 31:268–275
Dewas C, Fay M, Gougerot-Pocidalo MA, El-Benna J (2000) The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. J Immunol 165:5238–5244
Day BJ, Patel M, Calavetta L, Chang L-Y, Stamlet JS (1999) A mechanism of paraquat toxicity involving nitric oxide synthase. PNAS 96:12760–12765
Ebadi M, Sharma SK (2003) Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s disease. Antioxid Redox Signal 5:319–335
Duval DL, Miller DR, Collier J, Billings RE (1996) Characterization of hepatic nitric oxide synthase: identification as the cytokine-inducible form primarily regulated by oxidants. Mol Pharmacol 50:277–284
Margolis AS, Porasumphatana S, Rosen GM (2000) Role of paraquat in the uncoupling of nitric oxide synthase. Biochim Biophys Acta 1524:253–257
Guo Q, Tirosh O, Packer L (2001) Inhibitory effect of α-lipoic acid and its positively charged amide analogue on nitric oxide production in RAW 264.7 macrophages. Biochem Pharmacol 61:547–554
Shih C-L, Chi S-I, Chiu TH, Sun GY, Lin T-N (2001) Ethanol effects on nitric oxide production in cerebral pial cultures. Alcohol: Clin Exp Res 25:612–618
Noack H, Possel H, Rethfeldt C, Keilhoff G, Wolf G (1999) Peroxynitrite mediated damage and lowered superoxide tolerance in primary cortical glial cultures after induction of the inducible isoform of NOS. GLIA 28:13–24
Yamamoto F, Ohgari Y, Yamaki N, Kitajima S, Shimokawa O, Matsui H, Taketani S (2007) The role of nitric oxide in δ-aminolevulinic acid (ALA)-induced photosensitivity of cancerous cells. Biochem Biophys Res Commun 353:541–546
Connelly L, Madhani M, Hobbs AJ (2005) Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice. J Biol Chem 280:10040–10046
Mitsumoto A, Nakagawa Y (2001) DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic Res 35:885–893
Xia J, Simonyi AS, Sun GY (1999) Chronic ethanol and iron administration of iron content, neuronal nitric oxide synthase, and superoxide dismutase in rat cerebellum. Alcohol: Clin Exp Res 23:702–707
Gobbel GT, Chan TY-Y, Chan PH (1997) Nitric oxide- and superoxide-mediated toxicity in cerebral endothelial cells. J Pharmacol Exp Ther 282:1600–1607
Tomita M, Okuyama T, Ishikawa T, Hidaka K, Nohno T (2001) The role of nitric oxide in paraquat-induced cytotoxicity in the A549 lung carcinoma cell line. Free Radic Res 34:193–202
Shin CY, Choi J-W, Jang ES, Ju C, Kim W-K, Kim H-C, Choi C-R, Ko KH (2001) Dehydroepiandrosterone inhibits the death of immunostimulated rat C6 glioma cells deprived of glucose. Brain Res 922:267–275
Iravani MM, Leung CCM, Sadeghian M, Haddon CO, Rose S, Jenner P (2005) The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. Eur J Neurosci 22:317–330
Goralski KB, Renton KW (2004) Brain inflammation enhances 1-methyl-4-phenylpyridinium-evoked neurotoxicity in rats. Toxicol Appl Pharmacol 196:381–389
Schroder K, Sweet MJ, Hume DA (2006) Signal integration between IFNγ and TLR signaling pathways in macrophages. Immunobiology 211:511–524
Whitehead GS, Grasman KA, Kimmel EC (2003) Lung function and airway inflammation in rats following exposure to combustion products of carbon-graphite/epoxy composite material: comparison to a rodent model of acute lung injury. Toxicology 183:175–197
Shen S, Yu S, Binek J, Chalimoniuk M, Zhang X, Lo SC, Hannink M, Wu J, Fritsche K, Donato R, Sun GY (2005) Distinct signaling pathways for induction of type II NOS by IFNgamma and LPS in BV-2 microglial cells. Neurochem Int 47:298–307
Li W, Xia J, Sun GY (1999) Cytokine induction of iNOS and sPLA2 in immortalized astrocytes (DITNC): response to genistein and pyrrolidine dithiocarbamate. J Interf Cytok Res 19:121–127
Herrera AJ, Tomas-Camardiel M, Venero JL, Cano J, Machado A (2005) Inflammatory process as a determinant factor for the degeneration of substantia nigra dopaminergic neurons. J Neural Transm 112:111–119
Chandel NS, Trzyna WC, McClintock DS, Schumacker PT (2000) Role of oxidants in NF-κB activation and TNF-α gene transcription induced by hypoxia and endotoxin. J Immunol 165:1013–1021
West NP, Jungnitz H, Fitter JT, McArthur JD, Guzman CA, Walker MJ (2000) Role of phosphoglucomutase of Bordetella bronchiseptica in lipopolysaccharide biosynthesis and virulence. Infect Immun 68:4673–4680
Ling Z, Chang QA, Tong CW, Leurgans SE, Lipton JW, Carvey PM (2004) Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Exp Neurol 190:373–383
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. George DeVries.
Rights and permissions
About this article
Cite this article
Miller, R.L., James-Kracke, M., Sun, G.Y. et al. Oxidative and Inflammatory Pathways in Parkinson’s Disease. Neurochem Res 34, 55–65 (2009). https://doi.org/10.1007/s11064-008-9656-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9656-2