Abstract
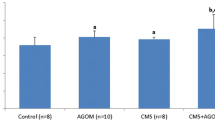
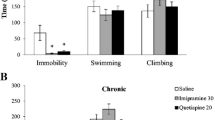
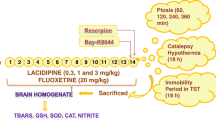
Venlafaxine is an approved antidepressant that is an inhibitor of both serotonin and norepinephrine transporters. Medical treatment with oral venlafaxine can be beneficial to depression due to reducing free radical production in the brain and medulla of depression- induced rats because oxidative stress may a play role in some depression. We investigated the effect of venlafaxine administration and experimental depression on lipid peroxidation and antioxidant levels in cortex brain, medulla and erythrocytes of rats. Thirty male wistar rats were used and were randomly divided into three groups. Venlafaxine (20 mg/kg) was orally supplemented to depression-induced rats constituting the first group for four week. Second group was depression-induced group although third group was used as control. Depressions in the first and second groups were induced on day zero of the study by chronic mild stress. Brain, medulla and erythrocytes samples were taken from all animals on day 28. Depression resulted in significant decrease in the glutathione peroxidase (GSH-Px) activity and vitamin C concentrations of cortex brain, glutathione (GSH) value of medulla although their levels were increased by venlafaxine administration to the animals of depression group. The lipid peroxidation levels in the three tissues and nitric oxide value in cortex brain elevated although their levels were decreased by venlafaxine administration. There were no significant changes in cortex brain vitamin A, erythrocytes vitamin C, GSH-Px and GSH, medulla vitamin A, GSH and GSH-Px values. In conclusion, cortex brain within the three tissues was most affected by oxidative stress although there was the beneficial effect of venlafaxine in the brain of depression-induced rats on investigated antioxidant defenses in the rat model. The treatment of depression by venlafaxine may also play a role in preventing oxidative stress.






Similar content being viewed by others
Abbreviations
- GSH-Px:
-
glutathione peroxidase
- GSH:
-
glutathione
- Hb:
-
hemoglobin
- LP:
-
lipid peroxidation
- MAO:
-
monoaminooxidase
- MDA:
-
malondialdehyde
- NO:
-
nitric oxide
- PUFAs:
-
polyunsaturated fatty acids
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- SSRIs:
-
Selective-serotonin reuptake inhibitors
References
Halliwell B, Gutteridge JMC (1999) Free radicals, other reactive species and disease. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine 3. Oxford University Press, New York, pp 639–645
Nazıroğlu M, Şimşek M, Kutlu M (2004) Moderate exercise with dietary vitamin C and E combination protects streptozotocin- induced oxidative damage to the blood and improves fetal outcomes in pregnant rats. Clin Chem Lab Med 42:511–517
Baydas G, Kutlu S, Nazıroğlu M, Canpolat S, Sandal S, Özcan M, Keleştimur H (2003) Inhibitory effects of melatonin on lipid peroxidation induced by intracerebroventricularly administered homocysteine. J Pineal Res 34:36–39
Nazıroğlu M (2006) Effects of physical exercise with a dietary vitamins C and E combination on oxidative stress in muscle, liver and brain of streptozotocin- induced diabetic pregnant rat. Braunstain MH (ed) Vitamin E: new research. Nova Science Publishers, Inc. NY, USA, pp 69–83
Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O (2001) Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 64:43–51
Takuma K, Baba A, Matsuda T (2004) Astrocyte apoptosis: implications for neuroprotection. Prog Neurobiol 72:111–127
Thase ME (2005) Bipolar depression: issues in diagnosis and treatment. Harv Rev Psychiatry 13:257–271
Maher P, Davis JB (1996) The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci 16:6394–6401
Whanger PD (2001) Selenium and the brain: a review. Nutr Neurosci 4:81–97
Nathan C, Xie OW (1994) Regulation of biosynthesis of nitric oxide. J Biol Chem 269:13725–13728
Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lonnqvist J (2004) Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry 161:567–569
Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, Christophe A (2000) Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord 58:241–246
Ozmen I, Naziroglu M, Alici HA, Sahin F, Cengiz M, Eren I (2007) Spinal morphine administration reduces the fatty acid contents in spinal cord and brain by ıncreasing oxidative stress. Neurochem Res 32:19–25
Andreasson A, Arborelius L, Erlanson-Albertsson C, Lekander M (2006) A putative role for cytokines in the impaired appetite in depression. Brain Behav Immun [Epub ahead of print]
Cyranowski JM, Marsland AL, Bromberger JT, Whiteside TL, Chang Y, Matthews KA (2006) Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun. [Epub ahead of print]
Kökçam I, Nazıroğlu M (2002) Effects of vitamin E supplementation on blood antioxidants levels in patients with Behçet’s disease. Clin Biochem 35:633–639
Bolden-Watson C, Richelson E (1993) Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci 52:1023–1029
Muth EA, Haskins JT, Moyer JA, Husbands GE, Nielsen ST, Sigg EB (1986) Antidepressant biochemical profile of the novel bicyclic compound Wy-45,030, an ethyl cyclohexanol derivative. Biochem Pharmacol 35:4493–4497
Grippo AJ, Moffitt JA, Johnson AK (2002) Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol 282:R1333–R1341
Grippo AJ, Beltz TG, Johnson AK (2003) Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav 78:703–710
Muscat R, Willner P (1992) Suppression of sucrose drinking by chronic mildunpredictable stress: a methodological analysis. Neurosci Biobehav Rev 16:507–517
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Anal Biochem 16:359–364
Cortas NK, Wakid NW (1990) Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem 36:1440–1443
Sedlak J, Lindsay RHC (1968) Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellmann’ s reagent. Anal Biochem 25:192–205
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-Phenol reagent. J Biol Chem 193:265–275
Cannan RK (1958) Hemoglobin (as cyanmethemoglobin) in blood. Clin Chem 4:246–251
Suzuki J, Katoh N (1990) A simple and cheap method for measuring vitamin A in cattle using only a spectrophotometer. Jpn J Vet Sci 52:1282–1284
Jagota SK, Dani HM (1982) A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem 127:178–182
Sierra-Honigmann MR, Murphy PA (1992) Suppression of interleukin-1 action by phospholipase-A2 inhibitors in helper T lymphocytes. Pept Res 5:258–261
Yamada M, Yamada M, Yamazaki S, Takahashi K, Nara K, Ozawa H, Yamada S, Kiuchi Y, Oguchi K, Kamijima K, Higuchi T, Momose K (2001) Induction of cysteine string protein after chronic antidepressant treatment in rat frontal cortex. Neurosci Lett 301:183–186
Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY (1999) Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res 22:275–291
Atmaca M, Tezcan E, Kuloglu M, Ustundag B, Tunckol H (2004) Antioxidant enzyme and malondialdehyde values in social phobia before and after citalopram treatment. Eur Arch Psychiatry Clin Neurosci 254:231–235
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of the paper was submitted in 1st Ion Channels and Oxidative Stress Congress, 14–16 September 2006, Isparta, Turkey.
Rights and permissions
About this article
Cite this article
Eren, İ., Nazıroğlu, M., Demirdaş, A. et al. Venlafaxine Modulates Depression-Induced Oxidative Stress in Brain and Medulla of Rat. Neurochem Res 32, 497–505 (2007). https://doi.org/10.1007/s11064-006-9258-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9258-9
Keywords
Profiles
- Ömer Çelik View author profile