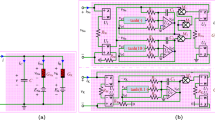
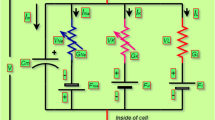
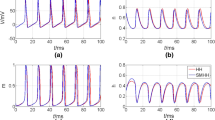
Abstract
Ion-channel variability has critical effect on the spike initiation and propagation in nervous system. Noise can play a constructive role leading to increased reliability or regularity of neuronal firing and spike propagation in the nervous system. In this paper we show that memristors can be considered as an electronic analogous of the Hodgkin–Huxley ion channels not only in terms of threshold switching effect but also in terms of stochastic behavior. In other words, memristor can also implement stochastic version of Hodgkin–Huxley equation. Switching effect in memristive devices is thermodynamically driven, which is stochastic in nature. We show that if the intrinsic stochastic behavior of memristor is taken into account, memristor based neuristor can also implement stochastic version of Hodgkin–Huxley axon model in generation of action potential. Ion channel variability in neurons can be modeled by intrinsic stochastic behavior of memristor. We incorporate noise in the memristor model by adding white Gaussian noise to the deterministic part of dynamical state evolution function of the memristor. We study the reliability of spike timing for spike train generated by memristor based neuristor in which the noise included memristor model is used. Also, the reliability of spike propagation along thin axons is discussed. A series connection of neuristors can be used as an axon in which neuristor acts as a node of Ranvier on an axon. Probabilistic nature of spike propagation on thin axons can be modeled using neuristor in which the variability nature of memristor is included.








Similar content being viewed by others
References
Faisal AA, White JA, Laughlin SB (2005) Ion-channel noise places limits on the miniaturization of the brain’s wiring. Curr Biol 15(12):1143–1149
Hille B (1970) Ionic channels in nerve membranes. Prog Biophys Mol Biol 21:1–32
Horikawa Y (1991) Noise effects on spike propagation in the stochastic Hodgkin-Huxley models. Biol Cybern 66(1):19–25
Schneidman E, Freedman B, Segev I (1998) Ion channel stochasticity may be critical in determining the reliability and precision of spike timing. Neural Comput 10(7):1679–1703
White JA, Rubinstein JT, Kay AR (2000) Channel noise in neurons. Trends Neurosci 23(3):131–137
Faisal AA, Selen LP, Wolpert DM (2008) Noise in the nervous system. Nat Rev Neurosci 9(4):292–303
Calvin WH, Stevens CF (1968) Synaptic noise and other sources of randomness in motoneuron interspike intervals. J Neurophysiol 31(4):574–587
Calvin WH, Stevens CF (1967) Synaptic noise as a source of variability in the interval between action potentials. Science 155:842–844
Strassberg AF, DeFelice LJ (1993) Limitations of the Hodgkin-Huxley formalism: effects of single channel kinetics on transmembrane voltage dynamics. Neural Comput 5(6):843–855
Rubinstein JT (1995) Threshold fluctuations in an N sodium channel model of the node of Ranvier. Biophys J 68(3):779
Blair EA, Erlanger J (1933) A comparison of the characteristics of axons through their individual electrical responses. Am J Physiol-Leg Content 106(3):524–564
Verveen AA (1962) Axon diameter and fluctuation in excitability. Acta Morphol Neerlando-Scand 5:79–85
Bryant HL, Segundo JP (1976) Spike initiation by transmembrane current: a white-noise analysis. J Physiol 260(2):279–314
Mainen ZF, Sejnowski TJ (1995) Reliability of spike timing in neocortical neurons. Science 268(5216):1503–1506
Galán RF, Ermentrout GB, Urban NN (2008) Optimal time scale for spike-time reliability: theory, simulations, and experiments. J Neurophysiol 99(1):277–283
Cecchi GA, Sigman M, Alonso JM, Martínez L, Chialvo DR, Magnasco MO (2000) Noise in neurons is message dependent. Proc Natl Acad Sci 97(10):5557–5561
Benzi R, Sutera A, Vulpiani A (1981) The mechanism of stochastic resonance. J Phys A: Math Gen 14(11):L453
Galán RF, Ermentrout GB, Urban NN (2005) Efficient estimation of phase-resetting curves in real neurons and its significance for neural-network modeling. Phys Rev Lett 94(15):158101
Talasila HS (2011) Effect of channel stochasticity on spike timing dependent plasticity (Doctoral dissertation)
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117(4):500–544
Groff JR, DeRemigio H, Smith GD (2009) Markov chain models of ion channels and calcium release sites. Stoch Methods Neurosci 24:29–64
Goldwyn JH, Shea-Brown E (2011) The what and where of adding channel noise to the Hodgkin-Huxley equations. PLoS Comput Biol 7(11):e1002247
Chua LO (1971) Memristor-the missing circuit element. Circuit Theory, IEEE Trans 18(5):507–519
Strukov DB, Snider GS, Stewart DR, Williams RS (2008) The missing memristor found. Nature 453(7191):80–83
Kim H, Sah MP, Yang C, Chua LO (2010). Memristor-based multilevel memory. In: Cellular nanoscale networks and their applications (CNNA), 2010 12th international workshop on IEEE, pp 1–6
Xia Q, Robinett W, Cumbie MW, Banerjee N, Cardinali TJ, Yang JJ, Williams RS (2009) Memristor—CMOS hybrid integrated circuits for reconfigurable logic. Nano Lett 9(10):3640–3645
Borghetti J, Snider GS, Kuekes PJ, Yang JJ, Stewart DR, Williams RS (2010) ‘Memristive’switches enable ‘stateful’logic operations via material implication. Nature 464(7290):873–876
Pershin YV, Ventra MD (2010) Practical approach to programmable analog circuits with memristors. Circuits Syst I: Regul Pap IEEE Trans 57(8):1857–1864
Jo SH, Chang T, Ebong I, Bhadviya BB, Mazumder P, Lu W (2010) Nanoscale memristor device as synapse in neuromorphic systems. Nano Lett 10(4):1297–1301
Chua L (2013) Memristor, Hodgkin-Huxley, and edge of chaos. Nanotechnology 24(38):383001
Pickett MD, Medeiros-Ribeiro G, Williams RS (2013) A scalable neuristor built with Mott memristors. Nat Mater 12(2):114–117
Jo SH, Kim KH, Lu W (2008) Programmable resistance switching in nanoscale two-terminal devices. Nano Lett 9(1):496–500
Savel’ev SE, Alexandrov AS, Bratkovsky AM, Williams RS (2011) Molecular dynamics simulations of oxide memristors: thermal effects. Appl Phys A 102(4):891–895
Simon M, Nardone M, Karpov VG, Karpov IV (2010) Conductive path formation in glasses of phase change memory. J Appl Phys 108(6):064514
Yang Y, Gao P, Gaba S, Chang T, Pan X, Lu W (2012) Observation of conducting filament growth in nanoscale resistive memories. Nat Commun 3:732
Russo U, Ielmini D, Redaelli A, Lacaita AL (2006) Intrinsic data retention in nanoscaled phase-change memories—Part I: Monte Carlo model for crystallization and percolation. Electron Devices IEEE Trans 53(12):3032–3039
Jo KH, Jung CM, Min KS, Kang SMS (2010) Self-adaptive write circuit for low-power and variation-tolerant memristors. Nanotechnol IEEE Trans 9(6):675–678
Kuekes PJ, Robinett W, Roth RM, Seroussi G, Snider GS, Williams RS (2006) Resistor-logic demultiplexers for nanoelectronics based on constant-weight codes. Nanotechnology 17(4):1052
Knag P, Lu W, Zhang Z (2014) A native stochastic computing architecture enabled by memristors. Nanotechnol IEEE Trans 13(2):283–293
Gaba S, Sheridan P, Zhou J, Choi S, Lu W (2013) Stochastic memristive devices for computing and neuromorphic applications. Nanoscale 5(13):5872–5878
Hamilton TJ, Afshar S, van Schaik A, Tapson J (2014) Stochastic electronics: a neuro-inspired design paradigm for integrated circuits. Proc IEEE 102(5):843–859
Pickett MD, Williams RS (2012) Sub-100 fJ and sub-nanosecond thermally driven threshold switching in niobium oxide crosspoint nanodevices. Nanotechnology 23(21):215202
Chudnovskii FA, Odynets LL, Pergament AL, Stefanovich GB (1996) Electroforming and switching in oxides of transition metals: the role of metal-insulator transition in the switching mechanism. J Solid State Chem 122(1):95–99
Longtin A (2003) Effects of noise on nonlinear dynamics. In: Beuter A (ed) Nonlinear dynamics in physiology and medicine. Springer, New York, pp 149–189
Mahnke R, Kaupuzs J, Lubashevsky I (2009) Physics of stochastic processes: how randomness acts in time. Wiley, Chichester
Al-Shedivat M, Naous R, Cauwenberghs G, Salama KN (2015) Memristors empower spiking neurons with stochasticity. IEEE J Emerg Sel Top Circuits Syst 5(2):242–253
Guan X, Yu S, Wong HS (2012) A SPICE compact model of metal oxide resistive switching memory with variations. IEEE Electron Device Lett 33(10):1405–1407
Koch C (1998) Biophysics of computation: information processing in single neurons. Oxford University Press, New York
Verveen AA (1960) On the fluctuation of threshold of the nerve fiber. In: Tower DP, Schadé JP (eds) Structure and function of the cerebral cortex. Elsevier, Amsterdam, pp 282–288
Pickett MD, Williams RS (2013) Phase transitions enable computational universality in neuristor-based cellular automata. Nanotechnology 24(38):384002
Faisal AA, Laughlin SB (2007) Stochastic simulations on the reliability of action potential propagation in thin axons. PLoS Comput Biol 3(5):e79–e79
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feali, M.S., Ahmadi, A. Realistic Hodgkin–Huxley Axons Using Stochastic Behavior of Memristors. Neural Process Lett 45, 1–14 (2017). https://doi.org/10.1007/s11063-016-9502-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11063-016-9502-5