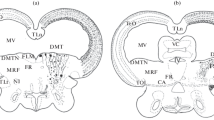
Using techniques of immunoperoxidase staining of proliferating cell nuclear antigen (PCNA) and TUNEL labeling of fragmented DNA, we studied sites of proliferation and apoptosis in the myelencephalon, cerebellum, tectum opticum, thalamus, and hypothalamus of the Amur sturgeon (Acipenser schrenckii). We found that the processes of proliferation and apoptosis are maintained in the brain of 3-year-old sturgeon individuals; the ratio of these processes in different cerebral regions varied significantly. The maximum intensity of proliferative activity was found in the periventricular zone of the myelencephalon (proliferation index, on average, 21.0 ± 1.3%). This fact allows us to consider this cerebral region a most important zone were adult neurogenesis occurs in the sturgeon. In the medial reticular formation, dorsal thalamic nuclei, inner fibrous layer of the tectum, and lateral hypothalamus, the maximum numbers of apoptotic elements were found. Therefore, these zones in the brain of the sturgeon correspond, apparently, to the regions where postmitotic neuroblasts are localized. In sensory centers (tectum and nuclei of the V, VII, and X nerves), significantly varying ratios of intensities of proliferation and apoptosis were found; this is indicative of dissimilar rates of growth and differentiation in visual and chemosensory centers of the sturgeon brain. The high proliferative activity in sensory and motor cerebral centers of the sturgeon allows us to hypothesize that a neotenic pattern is preserved in these CNS regions of adult sturgeons over a long period after the embryogenesis has been completed.
Similar content being viewed by others
References
G. K. H. Zupanc, K. Hinsch, and F.H. Gagr, “Proliferation, migration, neuronal differentiation and long-term survival of new cells in the adult zebrafish brain,” J. Comp. Neurol., 488, 290–319 (2005).
H. Grandel, J. Kaslin, J. Ganz, et al., “Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate,” Dev. Biol. 295, 263–277 (2006).
J. Soutschek and G. K. H. Zupanc, “Apoptosis in the cerebellum of adult teleost fish, Apteronotus leptorhynchus,” Dev. Brain Res., 97, 279–286 (1996).
К. Ampatzis and C. Dermon, “Sex differences in adult cell proliferation within the zebrafish (Danio rerio) cerebellum,” Eur. J. Neurosci., 25, 1030–1040 (2007).
E. N. Artyukhin, Sturgeonse (Ecology, Areal, and Phylogenesis) [in Russian], Publishing House of St. Petersburg University, St. Petersburg (2008).
G. A. Merkulov, Course of Pathological/Histological Technique [in Russian], Meditsina, Leningrad (1969).
G. K. H. Zupank, “Towards brain repair: Insights from teleost fish,” Seminars Cell Dev. Biol., 20, 683–690 (2009).
E. V. Pushchina, M. Yu. Fleishman, and S. S. Timoshin, “Proliferative zones in the brain of the Amur sturgeon fry. Interaction with neuromeres and migration of secondary matrix zones,” Russ. J. Dev. Biol., 38, 286–293 (2007).
G. K. H. Zupank, “Neurogenesis, cell death and regeneration in the adult gymnotiform brain,” J. Exp. Biol., 202, 1435–1446 (1999).
P. Ekström, C. Johnson, and L. Ohlin, “Ventricular proliferation zones in the brain of an adult teleost fish and their relation to neuromeres and migration (secondary matrix) zones,” J. Comp. Neurol., 436, 92–100 (2001).
R. S. Rajendran, U. M. Wellbrock, and G. K. H. Zupanc, “Apoptotic cell death, long-term persistence, and neuronal differentiation of aneuploid cells generated in the adult brain of teleost fish,” Dev. Neurobiol., 68, 1257–1268 (2008).
R. S. Rajendran, M. M. Zupanc, A. Lösche, et al., “Numerical chromosome variation and mitotic segregation defect in the adult brain of teleost fish,” Dev. Neurobiol., 67, 1334–1347 (2007).
S. K. Rehen, M. J. McConnell, D. Kaushal, et al., “Chromosomal variation in neurons of the developing and adult mammalian nervous system,” Proc. Natl. Acad. Sci. USA, 98, 13361–13366 (2001).
M. Wullimann and L. Puelles, “Postembryonic neural proliferation in the zebrafish forebrain and its relationship to prosomeric domains,” Anat. Embryol., 329, 329–348 (1999).
R. Bravo and H. MacDonald-Bravo, “Existence of two populations of cyclin/proliferating cell nuclear a ntigen during the cell cycle: association with DNA replication sites,” J. Cell Biol., 105, 1549–1554 (1987).
E. Candal, R. Anadon, W. DeGrip, and I. Rodríguez-Moldes, “Patterns of cell proliferation and cell death in the developing retina and optic tectum of brown trout,” Dev. Brain Res., 154, 101–119 (2005).
G. K. H. Zupanc and I. Horschke, “Proliferation zones in the brain of adult gymnotiform fish: a quantitative mapping study,” J. Comp. Neurol., 353, 213–233 (1995).
R. Brandstätter and K. Kotrschal, “Brain growth patterns in four European cyprinid fish species (Cyprinidae, Teleostei): roach (Rutilus rutilus), bream (Abramis brama), common carp (Cyprinus carpio) and sabre carp (Pelecus cultratus),” Brain, Behav., Evolut., 35, 195–211 (1990).
R. C. Marcus, C. L. Delaney, and S. S. Easter, “Neurogenesis in the visual system of embryonic and adult zebrafish (Danio rerio),” Vis. Neurosci., 16, 417–424 (1999).
R. Kubota, J. N. Hokoc, A. Moshiri, et al., “A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates,” Brain Res. Dev. Brain Res., 134, 31–41 (2002).
P. Rakic, “Neuroscience: immigration denied,” Nature, 427, 685–686 (2004).
H. Song, G. Kempermann, L. Overstreet Wadiche, et al., “New neurons in the adult mammalian brain: synaptogenesis and functional integration,” J. Neurosci., 25, 10366–10368 (2005).
P. M. Lledo, M. Alonso, and M. S. Grubb, “Adult neurogenesis and functional plasticity in neuronal circuits,” Nat. Rev. Neurosci., 7, 179–193 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Neirofiziologiya/Neurophysiology, Vol. 43, No. 4, pp. 315–331, July–August, 2011.
Rights and permissions
About this article
Cite this article
Puschina, E.V., Obukhov, D.K. Processes of Proliferation and Apoptosis in the Brain of the Amur Sturgeon. Neurophysiology 43, 271–286 (2011). https://doi.org/10.1007/s11062-011-9227-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-011-9227-z