Abstract
Purpose
Medulloblastoma (MB), a common and heterogeneous posterior fossa tumor in pediatric patients, presents diverse prognostic outcomes. To advance our understanding of MB’s intricate biology, the development of novel patient tumor-derived culture MB models with necessary data is still an essential requirement.
Methods
We continuously passaged PUMC-MB1 in vitro in order to establish a continuous cell line. We examined the in vitro growth using Cell Counting Kit-8 (CCK-8) and in vivo growth with subcutaneous and intracranial xenograft models. The xenografts were investigated histopathologically with Hematoxylin and Eosin (HE) staining and immunohistochemistry (IHC). Concurrently, we explored its molecular features using Whole Genome Sequencing (WGS), targeted sequencing, and RNA sequecing. Guided by bioinformatics analysis, we validated PUMC-MB1’s drug sensitivity in vitro and in vivo.
Results
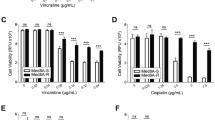
PUMC-MB1, derived from a high-risk MB patient, displayed a population doubling time (PDT) of 48.18 h and achieved 100% tumor growth in SCID mice within 20 days. HE and Immunohistochemical examination of the original tumor and xenografts confirmed the classification of PUMC-MB1 as a classic MB. Genomic analysis via WGS revealed concurrent MYC and OTX2 amplifications. The RNA-seq data classified it within the Group 3 MB subgroup, while according to the WHO classification, it fell under the Non-WNT/Non-SHH MB. Comparative analysis with D283 and D341med identified 4065 differentially expressed genes, with notable enrichment in the PI3K-AKT pathway. Cisplatin, 4-hydroperoxy cyclophosphamide/cyclophosphamide, vincristine, and dactolisib (a selective PI3K/mTOR dual inhibitor) significantly inhibited PUMC-MB1 proliferation in vitro and in vivo.
Conclusions
PUMC-MB1, a novel Group 3 (Non-WNT/Non-SHH) MB cell line, is comprehensively characterized for its growth, pathology, and molecular characteristics. Notably, dactolisib demonstrated potent anti-proliferative effects with minimal toxicity, promising a potential therapeutic avenue. PUMC-MB1 could serve as a valuable tool for unraveling MB mechanisms and innovative treatment strategies.




Similar content being viewed by others
Data availability
The data supporting the findings of this study can be obtained from the corresponding author upon a reasonable request.
References
Ni X, Li Z, Li X et al (2022) Socioeconomic inequalities in cancer incidence and access to health services among children and adolescents in China: a cross-sectional study. Lancet 400:1020–1032. https://doi.org/10.1016/S0140-6736(22)01541-0
Millard NE, De Braganca KC (2016) Medulloblastoma. J Child Neurol 31:1341–1353. https://doi.org/10.1177/0883073815600866
Taylor MD, Northcott PA, Korshunov A et al (2012) Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123:465–472. https://doi.org/10.1007/s00401-011-0922-z
Northcott PA, Robinson GW, Kratz CP et al (2019) Medulloblastoma. Nat Rev Dis Primers 5:1–20. https://doi.org/10.1038/s41572-019-0063-6
Northcott PA, Jones DTW, Kool M et al (2012) Medulloblastomics: the end of the beginning. Nat Rev Cancer 12:818–834. https://doi.org/10.1038/nrc3410
Ramaswamy V, Remke M, Bouffet E et al (2016) Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol 131:821–831. https://doi.org/10.1007/s00401-016-1569-6
Kumar V, Kumar V, McGuire T et al (2017) Challenges and recent advances in Medulloblastoma Therapy. Trends Pharmacol Sci 38:1061–1084. https://doi.org/10.1016/j.tips.2017.09.002
Gopalakrishnan V, Tao R-H, Dobson T et al (2015) Medulloblastoma development: tumor biology informs treatment decisions. CNS Oncol 4:79–89. https://doi.org/10.2217/cns.14.58
Becher OJ, Millard NE, Modak S et al (2017) A phase I study of single-agent perifosine for recurrent or refractory pediatric CNS and solid tumors. PLoS ONE 12:e0178593. https://doi.org/10.1371/journal.pone.0178593
Holzhauser S, Lukoseviciute M, Andonova T et al (2020) Targeting fibroblast growth factor receptor (FGFR) and phosphoinositide 3-kinase (PI3K) signaling pathways in Medulloblastoma Cell lines. Anticancer Res 40:53–66. https://doi.org/10.21873/anticanres.13925
Pócza T, Sebestyén A, Turányi E et al (2014) mTOR pathway as a potential target in a subset of human medulloblastoma. Pathol Oncol Res 20:893–900. https://doi.org/10.1007/s12253-014-9771-0
Lin F, de Gooijer MC, Hanekamp D et al (2017) PI3K-mTOR pathway inhibition exhibits Efficacy Against High-grade glioma in clinically relevant Mouse models. Clin Cancer Res 23:1286–1298. https://doi.org/10.1158/1078-0432.CCR-16-1276
Fròsina G, Profumo A, Marubbi D et al (2018) ATR kinase inhibitors NVP-BEZ235 and AZD6738 effectively penetrate the brain after systemic administration. Radiat Oncol 13:76. https://doi.org/10.1186/s13014-018-1020-3
Zhang L, Lei Y, Zhang Y et al (2018) Silencing of PRR11 suppresses cell proliferation and induces autophagy in NSCLC cells. Genes Dis 5:158–166. https://doi.org/10.1016/j.gendis.2017.12.003
Chen Y, Chen Y, Shi C et al (2018) SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 7:1–6. https://doi.org/10.1093/gigascience/gix120
Thompson EM, Keir ST, Venkatraman T et al (2017) The role of angiogenesis in Group 3 medulloblastoma pathogenesis and survival. Neuro Oncol 19:1217–1227. https://doi.org/10.1093/neuonc/nox033
Frühwald MC, O’Dorisio MS, Rush LJ et al (2000) Gene amplification in PNETs/medulloblastomas: mapping of a novel amplified gene within the MYCN amplicon. J Med Genet 37:501–509. https://doi.org/10.1136/jmg.37.7.501
Northcott PA, Shih DJH, Remke M et al (2012) Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol
Friedman HS, Burger PC, Bigner SH et al (1988) Phenotypic and genotypic analysis of a human medulloblastoma cell line and transplantable xenograft (D341 Med) demonstrating amplification of c-myc. Am J Pathol 130:472–484
Friedman HS, Burger PC, Bigner SH et al (1985) Establishment and characterization of the human Medulloblastoma Cell Line and Transplantable Xenograft D283 Med. J Neuropathology Experimental Neurol 44:592–605. https://doi.org/10.1097/00005072-198511000-00005
Casciati A, Tanori M, Manczak R et al (2020) Human medulloblastoma cell lines: investigating on Cancer Stem Cell-Like phenotype. Cancers 12:226. https://doi.org/10.3390/cancers12010226
Bunt J, Hasselt NE, Zwijnenburg DA et al (2011) Joint binding of OTX2 and MYC in promotor regions is associated with high gene expression in medulloblastoma. PLoS ONE 6:e26058. https://doi.org/10.1371/journal.pone.0026058
Lu Y, Labak CM, Jain N et al (2017) OTX2 expression contributes to proliferation and progression in myc-amplified medulloblastoma. Am J Cancer Res 7:647–656
Ballabio C, Anderle M, Gianesello M et al (2020) Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat Commun 11:583. https://doi.org/10.1038/s41467-019-13989-3
Adamson DC, Shi Q, Wortham M et al (2010) OTX2 is critical for the maintenance and progression of shh-independent medulloblastomas. Cancer Res 70:181–191. https://doi.org/10.1158/0008-5472.CAN-09-2331
Park AK, Lee JY, Cheong H et al (2019) Subgroup-specific prognostic signaling and metabolic pathways in pediatric medulloblastoma. BMC Cancer 19:571. https://doi.org/10.1186/s12885-019-5742-x
Gao R, Zhang R, Zhang C et al (2018) LncRNA LOXL1-AS1 promotes the proliferation and Metastasis of Medulloblastoma by activating the PI3K/AKT pathway. Analytical cellular pathology. https://doi.org/10.1155/2018/9275685. Amsterdam) 2018:9275685
Dudu V, Able RAJ, Rotari V et al (2012) Role of epidermal growth factor-triggered PI3K/Akt signaling in the Migration of Medulloblastoma-Derived cells. Cell Mol Bioeng 5:502–413. https://doi.org/10.1007/s12195-012-0253-8
Leroy C, Ramos P, Cornille K et al (2016) Activation of IGF1R/p110β/AKT/mTOR confers resistance to α-specific PI3K inhibition. Breast Cancer Res 18:41. https://doi.org/10.1186/s13058-016-0697-1
Elkabets M, Vora S, Juric D et al (2013) mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med 5:196ra99. https://doi.org/10.1126/scitranslmed.3005747
Le X, Antony R, Razavi P et al (2016) Systematic functional characterization of resistance to PI3K inhibition in breast Cancer. Cancer Discov 6:1134–1147. https://doi.org/10.1158/2159-8290.CD-16-0305
Eckerdt F, Clymer J, Bell JB et al (2019) Pharmacological mTOR targeting enhances the antineoplastic effects of selective PI3Kα inhibition in medulloblastoma. Sci Rep 9:12822. https://doi.org/10.1038/s41598-019-49299-3
Glaviano A, Foo ASC, Lam HY et al (2023) PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer 22:138. https://doi.org/10.1186/s12943-023-01827-6
Acknowledgements
None.
Funding
This work was supported by “CAMS Innovation Fund for Medical Sciences (CIFMS; 2021-1-I2M-053 to Yuqin Liu)”.
Author information
Authors and Affiliations
Contributions
MG and YQL conceived and designed the study. SZW and DZ performed the experiments, analyzed the data, and wrote the manuscript. JLW and ZLY participated cell culture. XJP, HLS and YQJ provided patient tissue and related information. XCB and YHH performed STR profiling and species identification. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of Institute of Basic Medical Sciences, CAMS, and Ethics Committee of Institute of Beijing Children’s Hospital, with the informed consent of parents. All in vivo experiments were conducted in accordance with protocols approved by the Peking Union Medical College Institutional Animal Care and Use Committee and performed in accordance with institutional guidelines and regulations. The ethical approval number for animal experiments is ACUC-A02-2023-085. Mice had free access to food and water in a 12 h light:12 h dark cycle according to standard guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Zhang, D., Wang, J. et al. PUMC-MB1 is a novel group 3 medulloblastoma preclinical model, sensitive to PI3K/mTOR dual inhibitor. J Neurooncol 168, 139–149 (2024). https://doi.org/10.1007/s11060-024-04655-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-024-04655-w