Abstract
Purpose
This review compares reirradiation (reRT), systemic therapy and combination therapy (reRT & systemic therapy) with regards to overall survival (OS), progression-free survival (PFS), adverse effects (AEs) and quality of life (QoL) in patients with recurrent high-grade glioma (rHGG).
Methods
A search was performed on PubMed, Scopus, Embase and CENTRAL. Studies reporting OS, PFS, AEs and/or QoL and encompassing the following groups were included; reirradiation vs systemic therapy, combination therapy vs systemic therapy, combination therapy vs reRT, and bevacizumab-based combination therapy vs reRT with/without non-bevacizumab-based systemic therapy. Meta-analyses were performed utilising a random effects model. Certainty of evidence was assessed using GRADE.
Results
Thirty-one studies (three randomised, twenty-eight non-randomised) comprising 2084 participants were included. In the combination therapy vs systemic therapy group, combination therapy improved PFS (HR 0.57 (95% CI 0.41–0.79); low certainty) and OS (HR 0.73 (95% CI 0.56–0.95); low certainty) and there was no difference in grade 3 + AEs (RR 1.03 (95% CI 0.57–1.86); very low certainty). In the combination therapy vs reRT group, combination therapy improved PFS (HR 0.52 (95% CI 0.38–0.72); low certainty) and OS (HR 0.69 (95% CI 0.52–0.93); low certainty). In the bevacizumab-based combination therapy vs reRT with/without non-bevacizumab-based systemic therapy group, adding bevacizumab improved PFS (HR 0.46 (95% CI 0.27–0.77); low certainty) and OS (HR 0.42 (95% CI 0.24–0.72; low certainty) and reduced radionecrosis (RR 0.17 (95% CI 0.06–0.48); low certainty).
Conclusions
Combination therapy may improve OS and PFS with acceptable toxicities in patients with rHGG compared to reRT or systemic therapy alone. Particularly, combining bevacizumab with reRT prophylactically reduces radionecrosis.
Registration: CRD42022291741.
Similar content being viewed by others
Introduction
High-grade gliomas (HGG) consist of glioblastoma multiforme (GBM) and anaplastic gliomas (anaplastic astrocytoma and anaplastic oligodendroglioma) [1]. Most HGGs are managed with a multimodal approach, incorporating maximal safe surgical resection, postoperative radiotherapy and temozolomide [2]. Despite this, HGGs have a poor prognosis with a median overall survival (OS) of 15 months and nearly all patients experiencing tumour recurrence [3]. Treatment options for recurrent HGG (rHGG) are limited and include reoperation, reirradiation (reRT), second-line chemotherapy, or a combination of these [4].
Notably, 90% of recurrences occur within 2 cm of the original tumour site, suggesting the need for improved local control [5]. To control local progression, surgical resection is one treatment option, with a median reported OS of 9.7 months. However, only 20–30% of patients with recurrent or progressive disease have resectable lesions, and reoperation is often limited by performance status and diffuse, infiltrative disease involving eloquent areas [4].
ReRT is another localised treatment option for recurrent or progressive disease. It generally benefits patients with a good performance status (KPS > 60), localised/unifocal disease, and a time interval between initial radiation and reRT of at least 6 months [6, 7]. Retrospective data suggests that reRT is safe and provides improved local control [8], with a median reported OS of 7.5–16 months [6] and an OS-12 rate of 36% in recurrent GBM (rGBM) [9]. ReRT is controversial given the tendency for in/near-field recurrences, and hence the increased risk for radiation necrosis (RN) from cumulative dose. The reported incidence of RN following reRT is 0–31.3% [10]. Risk factors associated with RN include fractionation schedule, dose (cumulative EQD2), treatment volume, time interval between initial radiation and reRT, and concomitant systemic therapy. Three external beam radiotherapy techniques are used based on fractionation schedule; stereotactic radiosurgery (SRS), hypofractionated stereotactic radiotherapy (HFSRT), and conventionally fractionated radiotherapy (conventional RT) [7].
Second-line chemotherapy is commonly required for durable tumour control. Literature reports a median OS of 6–9 months in patients with rGBM treated with salvage chemotherapy. No single agent or combination of systemic therapies has demonstrated superiority to the others [11,12,13]. Bevacizumab has demonstrated efficacy in delaying tumour progression, albeit without an OS improvement, and hence has been approved by the FDA for treatment of rGBM [14]. Nevertheless, most patients with rHGG progress on bevacizumab after a median time of 3–5 months [15].
Several retrospective studies report the safety and efficacy of combining reRT with bevacizumab [16]. In a network meta-analysis of treatment options for progressive or rGBM, McBain et al. found limited evidence suggesting reRT with or without bevacizumab may improve survival in select individuals [17]. Bevacizumab is further postulated to reduce radioresistance and to lower the incidence of RN [18]. Temozolomide has also been studied in combination with reRT in rHGG due to its radio-sensitizing effect [19]. However, its value in this setting is uncertain given its widespread use in initial treatment and possible subsequent resistance [20].
Combination therapy with localised treatment and systemic therapy allows for the simultaneous targeting of macroscopic, microscopic, and diffuse disease, and hence may result in improvements in OS and progression-free survival (PFS). However, as the treatment of rHGG is of palliative intent, cumulative treatment-related toxicities must be balanced with the potential impact of disease progression on quality of life (QoL). This review aims to compare the impact of reRT, systemic therapy and combination therapy (reRT & systemic therapy) with regards to OS, PFS, adverse effects (AEs), and quality of life (QoL) in patients with rHGG.
Methods
This review was conducted in accordance with PRISMA guidelines.
Search strategy
A search was performed on PubMed, Scopus, Embase and CENTRAL on 18 March 2022. A repeat search was conducted on 1 February 2023. The search was limited to studies published from 2010, with no limits on language.
The following Boolean search was utilised;
(“radiation” OR “radiotherapy” OR “radio-therapy” OR “radio therapy” OR “irradiation” OR “reirradiation” OR “re-irradiation” OR “re irradiation” OR “RT” OR “stereotactic” OR “radiosurgery” OR “radio-surgery” OR “radio surgery”) AND (“glioblastoma” OR “GBM” OR “high grade glioma” OR “high grade gliomas” OR “high-grade glioma” OR “high-grade gliomas” OR “malignant glioma” OR “malignant gliomas”) AND (“recurrent” OR “recurrence” OR “progressive”).
Eligibility criteria
Studies reporting OS, PFS, AEs and/or QoL in patients with rHGG, and encompassing the following groups were included; reirradiation vs systemic therapy, combination therapy vs systemic therapy, combination therapy vs reirradiation, and bevacizumab-based combination therapy vs reirradiation ± non-bevacizumab-based systemic therapy. Studies were included only if patients received external beam radiotherapy.
Studies were excluded if they were single-arm studies, reviews, case reports, conference abstracts, animal studies or in-vitro studies, or if they did not strictly encompass the comparative treatment groups or report any outcomes of interest. Studies incorporating patients receiving brachytherapy and patients with low grade gliomas or other non-glial CNS tumours were also excluded.
The first author (R.M) independently conducted title/abstract screening. Both first and second authors (R.M and D.X) performed full text screening individually. Disagreements were resolved by consensus. The reference lists of included manuscripts were examined to identify additional articles.
Data collection
Data was extracted on study characteristics, demographics, tumour characteristics, intervention characteristics, and outcome measures of interest (OS, PFS, AEs (RN (any grade), CTCAE Grade 3 + toxicities, treatment-related deaths), QoL) by the first author (R.M) and cross-checked by the second author (D.X). To maintain consistent definitions, OS and PFS were only collected for studies which measured these outcomes from recurrence or retreatment.
Quality assessment
Risk of bias (RoB) was independently assessed by first and second authors (R.M & D.X) using the Cochrane RoB 2 tool [21] for randomised control trials (RCTs) and the ROBINS-I tool [22] for non-randomised studies. Differences between authors were resolved with discussion on completion. Publication bias was assessed through the visual inspection of funnel plots generated using the RevMan 5.4 software.
Synthesis methods
Only studies of low to moderate RoB were included in the meta-analyses, while studies of serious or critical RoB were excluded. Meta-analyses were performed for outcome measures of interest utilising the RevMan 5.4 software. Meta-analyses could not be conducted for QoL due to insufficient reporting. The logHR and SE(logHR) for OS and PFS, and logRR and SE(logRR) for AEs were extracted or estimated if not reported [23]. Data was pooled by each comparative treatment group using the generic inverse variance method, and the DerSimionian and Laird random effects model was utilised given expected heterogeneity. A forest plot was generated for each outcome measure within each comparative treatment group. Statistical significance was defined as p < 0.05 and 95% confidence intervals were reported. A subset analysis was performed for only RCTs if ≥ 2 studies were available. Further subset analysis was also performed for GBM-only studies.
GRADE approach
The overall certainty of evidence was assessed for each outcome using the Grading, Recommendations, Assessment, Development and Evaluation (GRADE) approach [24].
Results
Study selection
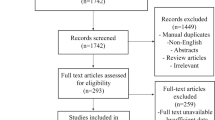
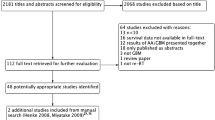
As demonstrated in Supplementary Material; Fig. 1, 12,244 studies were identified in the initial search, with 7338 studies remaining after removing duplicates. 7279 studies were excluded on title and abstract screening resulting in 59 articles for full-text screen. Of these articles, 28 were included; 9 were excluded as they were non-comparative, 19 were excluded as they did not strictly encompass the comparative treatment groups, 2 were excluded as they included patients treated with brachytherapy, and 1 was excluded as it included patients with low grade gliomas or other non-glial CNS tumours. 3 additional articles [25,26,27] were included after an updated search was conducted on 1 February 2023, resulting in a total of 31 articles. No additional articles were included on examination of reference lists of included studies.
Study and treatment characteristics
Study and treatment characteristics are summarised in Tables 1 and 2. Thirty-one studies (three RCTs, one matched-case control study, twenty-seven cohort studies) comprising 2084 participants were included. Participants incorporated 1076 males, 739 females, and 269 individuals of unspecified sex. 1593 participants had WHO Grade IV tumours, 210 had WHO Grade III tumours, and 281 had non-specified HGGs. 4 studies [28,29,30,31] comprised the reirradiation vs systemic therapy group, 7 studies [26, 27, 31,32,33,34,35] encompassed the combination therapy vs systemic therapy group, 17 studies [31, 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] comprised the combination therapy vs reirradiation group, and 8 studies [18, 25, 49,50,51,52,53,54] encompassed the bevacizumab-based combination therapy vs reirradiation with/without non-bevacizumab-based systemic therapy group.
Risk of bias
The RoB assessment is demonstrated in the Supplementary Material; Figs. 2, 3, 4, 5. The 3 RCTs [27, 33, 37] were of low RoB. Of the 28 non-randomised studies, 19 were of moderate RoB [18, 25, 28, 29, 31, 32, 34, 35, 38, 40, 43, 45, 48,49,50,51,52,53,54], and 9 were of serious RoB (and hence excluded from meta-analyses) [26, 30, 36, 39, 41, 42, 44, 46, 47].
Treatment outcomes & meta-analyses: rHGG
Reirradiation vs systemic therapy
In the reRT group, the median PFS ranged from 3.6 to 7.7 months, while the median OS ranged from 4.3 to 9.5 months. In the systemic therapy group, the median PFS ranged from 2.3 to 4.3 months, while the median OS ranged from 5.3 to 7.3 months. No grade 3–5 toxicities were reported in this group (Table 2).
There was no difference in PFS (2 studies [28, 31], 185 participants; HR 0.87 (95% CI 0.61–1.22), p = 0.41, I2 = 0%; very low certainty) and OS (3 studies [28, 29, 31], 237 participants; HR 0.94 (95%CI 0.67–1.31), p = 0.70, I2 = 0%; very low certainty) (Fig. 1). A meta-analysis could not be conducted for AEs due to insufficient reporting.
Combination therapy vs systemic therapy
In the combination therapy group, the median PFS ranged from 5.1 to 12 months, while the median OS ranged from 7.2 to 16 months. In the systemic therapy group, the median PFS ranged from 1.8 to 4.8 months, while the median OS ranged from 4.8 to 9.7 months. In the combination therapy group rates of grade 3 + AEs ranged from 2.1 to 33.3% while in the systemic therapy group rates ranged from 0 to 23.8%. 1 treatment-related death was reported with combination therapy (Table 2).
Combination therapy improved PFS (5 studies [27, 31,32,33,34], 402 participants; HR 0.57 (95% CI 0.41–0.79), p = 0.0008, I2 = 55%; low certainty) and OS (6 studies [27, 31,32,33,34,35], 537 participants; HR 0.73 (95% CI 0.56–0.95), p = 0.02, I2 = 35%; low certainty), and there was no difference in grade 3 + toxicities (4 studies [27, 33,34,35], 398 participants; RR 1.03 (95% CI 0.57–1.86), p = 0.92, I2 = 21%; very low certainty) (Fig. 2).
Subset analyses of studies comparing combination therapy with bevacizumab-based systemic therapy to bevacizumab-based systemic therapy alone are demonstrated in Supplementary Material; Fig. 6. Subset analyses of only RCTs are demonstrated in Supplementary Material; Fig. 7.
Combination therapy vs reirradiation
In the combination therapy group, the median PFS ranged from 4.3 to 14.9 months, while the median OS ranged from 4.8 to 17.9 months. In the reRT group, the median PFS ranged from 2 to 6.7 months, while the median OS ranged from 4 to 14.3 months. In the combination therapy group, rates of grade 3 + AEs ranged from 0 to 33%, while in the reRT group rates ranged from 0 to 50.0%. RN rates ranged from 0 to 9.5%. Two treatment-related deaths were reported in the combination therapy group (Table 2).
Combination therapy improved PFS (4 studies [31, 37, 40, 49], 236 participants; HR 0.52 (95% CI 0.38–0.72), p < 0.0001, I2 = 0%; low certainty) and OS (7 studies [31, 37, 38, 40, 43, 49, 50], 471 participants; HR 0.69 (95% CI 0.52–0.93), p = 0.02, I2 = 18%; low certainty) (Fig. 3).
Bevacizumab-based combination therapy vs reirradiation with/without non-bevacizumab-based systemic therapy
In the bevacizumab-based combination therapy group, the median PFS ranged from 5 to 14.9 months, while the median OS ranged from 7.9 to 17.9 months. In the reRT without bevacizumab group, the median PFS ranged from 2.1 to 6.7 months, while the median OS ranged from 3.9 to 14.3 months. RN rates in the bevacizumab-based combination therapy group ranged from 0 to 9%, while the RN rates in the reRT without bevacizumab group ranged from 13.5 to 66.7%. Rates of grade 3 + AEs ranged from 0 to 10% in the bevacizumab-based combination therapy group while they ranged from 0 to 50% in the reRT without bevacizumab group. 1 treatment-related death was reported in the bevacizumab-based combination therapy group (Table 2).
Combining reRT with bevacizumab-based systemic therapy improved PFS (2 studies [49, 53], 104 participants; HR 0.46 (95% CI 0.27–0.77), p = 0.003, I2 = 0%; low certainty) and OS (5 studies [25, 49, 50, 52, 53], 256 participants; HR 0.42 (95% CI 0.24–0.72), p = 0.001, I2 = 38%; low certainty), while reducing RN (5 studies [18, 50, 51, 53, 54], 353 participants; RR 0.17 (95% CI 0.06–0.48), p = 0.0008, I2 = 25%; low certainty) (Fig. 4).
Subset analysis: rGBM
In the reRT vs systemic therapy group, there was no difference in PFS (2 studies [28, 31], 185 participants; HR 0.87 (95% CI 0.61–1.22), p = 0.41, I2 = 0%) and OS (3 studies [28, 29, 31], 237 participants; HR 0.94 (95% CI 0.67–1.31), p = 0.70, I2 = 0%) (Supplementary Material; Fig. 8). In the combination therapy vs systemic therapy group, combination therapy improved PFS (2 studies [27, 31], 229 participants; HR = 0.66 (95% CI 0.49–0.91), p = 0.01, I2 = 15%), although there was no difference in OS (2 studies [27, 31], 229 participants; HR 0.90 (95% CI 0.62–1.32), p = 0.60, I2 = 11%) (Supplementary Material; Fig. 9). In the combination therapy vs reRT group, combination therapy improved PFS (4 studies [31, 37, 40, 49], 229 participants; HR 0.52 (95% CI 0.38–0.72), p < 0.0001, I2 = 0%) and OS (5 studies [31, 37, 38, 40, 49], 257 participants; HR 0.55 (95% CI 0.39–0.76), p < 0.0003, I2 = 0%) (Supplementary Material; Fig. 10). Combining reRT with bevacizumab-based systemic therapy improved PFS (2 studies [49, 53], 104 participants; HR 0.46 (95% CI 0.27–0.77), p = 0.003, I2 = 0%) and OS (4 studies [25, 49, 52, 53], 189 participants; HR 0.34 (95% CI 0.21–0.55), p < 0.00001, I2 = 0%) (Supplementary Material; Fig. 11).
Publication bias
Funnel plots are presented in the Supplementary Material; Figs. 12, 13, 14, 15. On visual inspection, there is low evidence of bias in most funnel plots. There is some concern for publication bias for toxicities (RN) in the bevacizumab-based combination therapy vs reirradiation with/without non-bevacizumab-based systemic therapy group (Supplementary Material; Fig. 15).
GRADE approach
The certainty of evidence assessment is summarised in Supplementary Material; Table 1.
Discussion
Compared to reRT alone, combination therapy improved OS and PFS. While there was insufficient information to conduct a meta-analysis comparing AEs, Kazmi et al. reported no significant differences in toxicity between reRT alone and combination therapy (5% vs 9% respectively, p = 0.22) [9]. Further RCTs are required to confirm the survival benefit and safety of combination therapy compared to reRT alone.
Particularly, the addition of bevacizumab to reRT with/without non-bevacizumab-based systemic therapy improved OS and PFS and reduced RN. Grade 3 + AEs were also lower with bevacizumab compared to without (0–10% vs 0–50%, respectively), largely secondary to decreased rates of Grade 3 + RN. These findings differ from the AVAglio [55] and RTOG 0825 [56] trials which explored the supplementation of the Stupp protocol [2] with bevacizumab in primary GBM. Both RCTs found bevacizumab resulted in a PFS improvement and a modest increase in grade 3 + AEs. However, as neither trial demonstrated an OS benefit, bevacizumab is not routinely used for primary GBM. Bevacizumab is, however, commonly utilised for recurrent GBM in the absence of proven OS benefits due to reported PFS improvements and steroid sparing effects, both which are postulated to improve QoL [14]. This review supports further investigation into the addition of bevacizumab to reRT in the recurrent HGG context given the demonstrated OS and PFS improvements and lower rates of grade 3 + AEs secondary to decreased rates of grade 3 + RN.
The addition of bevacizumab to reRT may also allow for safe dose escalation and for the treatment of larger volume disease due to its radioprotective properties. While bevacizumab is routinely used for the treatment of RN [57], it is not regularly used as a prophylactic agent [18]. In 2012, Sminia and Mayer found that RN occurred with a cumulative EQD2 dose > 100 Gy for conventional RT, > 105 Gy for HFSRT, and > 135 Gy for SRS. [58] In this review, 17 studies reported RN rates ranging from 0 to 9.5% (Supplementary Material; Table 2). 9 of these 17 studies escalated their cumulative EQD2 (a/b = 2) beyond Sminia and Mayer’s recommendation. Importantly, of these 9 studies, the rate of RN in the subset of patients that received bevacizumab with reRT was 0%, while the rate of RN in the subset of patients that did not receive bevacizumab ranged from 4.3 to 25.0%. Hence, bevacizumab may allow for safe dose escalation with acceptable rates of RN. Further studies are required to confirm if dose escalation confers improved local control or survival outcomes. RN is also a concern in the treatment of large volume disease. In 2021, Minniti et al. recommended SRS or high dose HFSRT (≥ 5 Gy/#) for smaller volume tumours (≤ 15 cc), high-dose HFSRT (≥ 5 Gy/#) for 8.5–34 cc tumours, and conventional RT or moderately HFSRT (1.8–3.5 Gy/#) for larger tumours (33–145 cc) to appreciate a low risk of RN [7]. In this review, 10 of 17 studies reporting RN rates also reported median PTV (Supplementary Material; Table 2). Of these 10 studies, 8 had median PTV > 34 cc. Importantly, in these 8 studies, the rate of RN in the subset of patients that received bevacizumab with reRT ranged from 0 to 4.8%, while the rate of RN in the subset of patients that did not receive bevacizumab ranged from 0 to 66.7%. Notably, all 8 studies utilised conventional RT or moderately HF-SRT, as recommended by Minniti et al. for larger volume tumours. Hence, reRT with concomitant bevacizumab may allow for the safer treatment of larger volume disease with acceptable rates of RN, particularly if the appropriate fractionation schedule is utilised.
Compared to systemic therapy alone, combination therapy (particularly with bevacizumab-based systemic therapy) improved OS and PFS with no difference in grade 3 + AEs. Two RCTs investigated bevacizumab with/without reRT in rHGG [27, 33]. Tsien et al. [27] compared bevacizumab with/without HFSRT (35 Gy/10#) in patients with bevacizumab-naïve rGBM. The study found that HFSRT improved PFS and 6-month PFS rates, though no improvements in OS were observed. However, due to low accrual, the study was amended to extend eligibility resulting in the inclusion of a large number of patients less likely to experience a survival benefit from focal HFSRT. Bergman et al. [33] compared bevacizumab-based systemic therapy with/without intervening HFSRT (32 Gy/4#) in patients with bevacizumab-resistant rHGG. Patients assigned to intervening HFSRT reported improved PFS and a nonsignificant improvement in OS, despite the study also failing to meet accrual goals to detect an OS difference. Interestingly, while Bergman et al. targeted FLAIR abnormalities in their CTV delineation, Tsien et al. did not. FLAIR abnormalities are non-enhancing regions that likely contain microscopic disease. Studies targeting FLAIR abnormalities have demonstrated improved locoregional control, suggesting that the deterioration of patients with rGBM may be due to insufficient reRT dose to these regions [59]. Further RCTs comparing systemic therapy with combination therapy, particularly with bevacizumab-based systemic therapy, are required. Importantly, the limitations of previous RCTs must be addressed; namely the inadequate accrual of appropriate patients, and the exclusion of FLAIR abnormalities from CTV delineation. Studies comparing QoL and neurocognitive function are needed as well.
Subset analyses of rGBM-only studies demonstrated similar improvements in OS and PFS with combination therapy across all comparative treatment groups, though no significant OS benefit was observed compared to systemic therapy alone. These findings are of particular clinical significance as there is no widely accepted standard-of-care for this patient cohort with the poorest prognosis [17]. Hence, further RCTs comparing combination therapy to systemic therapy or reirradiation alone in patients with rGBM are especially warranted.
This review has some limitations. There were insufficient studies to conduct a meta-analysis on QoL, and the impact of resection on survival could not be ascertained. Studies were also mainly of retrospective cohort methodology, hence conferring a greater risk of confounding and selection bias potentially favouring combination treatment. Furthermore, most studies incompletely reported molecular information (IDH/MGMT) and were conducted prior to the changes in WHO glioma classification in 2021. Of note, grading largely informs the management approach at initial diagnosis and relapse, and MGMT methylation status is a vital prognosticator in an era where most gliomas are treated with alkylating agents [4]. Further RCTs are therefore required to address these limitations and confirm this review’s findings. Additionally, the diagnosis of tumour progression/recurrence varied between studies; radiological diagnosis vs biopsy-proven. Notably, it is difficult to differentiate tumour progression with treatment-related changes using conventional MRI [60].
Conclusion
This review found that combination therapy may improve OS and PFS with acceptable toxicity in select patients with rHGG compared to reirradiation or systemic therapy alone. Hence, further RCTs are warranted although the limitations of previous RCTs must be addressed; namely the inadequate accrual of appropriate patients to detect an OS difference, and the exclusion of FLAIR abnormalities from CTV delineation. Additional studies comparing QoL and neurocognitive function are needed as well. This review also found that the addition of bevacizumab to reRT reduced RN and may allow for safer dose escalation and treatment of larger volume disease. Further studies are required to determine if dose escalation confers improved local control or survival outcomes.
Data availability
Data will be made available by the corresponding author upon reasonable request.
References
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Ostrom QT, Gittleman H, Truitt G et al (2018) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol 20:iv1–iv86
Weller M, van den Bent M, Preusser M et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186
Martínez-Carrillo M, Tovar-Martín I, Zurita-Herrera M et al (2014) Salvage radiosurgery for selected patients with recurrent malignant gliomas. Biomed Res Int 2014:657953
Scoccianti S, Francolini G, Carta GA et al (2018) Re-irradiation as salvage treatment in recurrent glioblastoma: a comprehensive literature review to provide practical answers to frequently asked questions. Crit Rev Oncol Hematol 126:80–91
Minniti G, Niyazi M, Alongi F, Navarria P, Belka C (2021) Current status and recent advances in reirradiation of glioblastoma. Radiat Oncol 16(1):36
Combs SE, Niyazi M, Adeberg S et al (2018) Re-irradiation of recurrent gliomas: pooled analysis and validation of an established prognostic score-report of the Radiation Oncology Group (ROG) of the German Cancer Consortium (DKTK). Cancer Med 7:1742–1749
Kazmi F, Soon YY, Leong YH, Koh WY et al (2019) Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. J Neurooncol 142(1):79–90
Shanker M, Chua B, Bettington C et al (2019) Re-irradiation for recurrent high-grade gliomas: a systematic review and analysis of treatment technique with respect to survival and risk of radionecrosis. Neurooncol Pract 6(2):144–155. https://doi.org/10.1093/nop/npy019
Reardon DA, Omuro A, Brandes AA et al (2017) OS10.3 Randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro Oncol 19:iii21
Wick W, Gorlia T, Bendszus M et al (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377(20):1954–1963
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Li Y, Ali S, Clarke J, Cha S (2017) Bevacizumab in recurrent glioma: patterns of treatment failure and implications. Brain Tumor Res Treat 5:1–9
Flieger M, Ganswindt U, Schwarz SB et al (2014) Re-irradiation and bevacizumab in recurrent high-grade glioma: an effective treatment option. J Neurooncol 117:337–345
McBain C, Lawrie TA, Rogozińska E et al (2021) Treatment options for progression or recurrence of glioblastoma: a network meta-analysis. Cochrane Database Syst Rev 5(1):CD013579
Fleischmann DF, Jenn J, Corradini S et al (2019) Bevacizumab reduces toxicity of reirradiation in recurrent high-grade glioma. Radiother Oncol 138:99–105
Combs SE, Bischof M, Welzel T et al (2008) Radiochemotherapy with temozolomide as re-irradiation using high precision fractionated stereotactic radiotherapy (FSRT) in patients with recurrent gliomas. J Neuro-Oncol 89:205–210
Messaoudi K, Clavreul A, Lagarce F (2015) Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and stra-tegies to overcome resistance of glioblastoma to temozolomide. Drug Discov Today 20:899–905
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Sterne JAC, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Tierney JF, Stewart LA, Ghersi D et al (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16. https://doi.org/10.1186/1745-6215-8-16
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926. https://doi.org/10.1136/bmj.39489.470347
Helis CA, Prim SN, Cramer CK et al (2022) Clinical outcomes of dose-escalated re-irradiation in patients with recurrent high-grade glioma. Neurooncol Pract 9(5):390–401
Lee YP, Jung HA, Lee MS et al (2022) Bevacizumab plus irinotecan with or without gamma knife radiosurgery after failure of concurrent chemo-radiotherapy for high-grade glioma. J Neuro-Oncol 156(3):541–549
Tsien CI, Pugh SL, Dicker AP et al (2022) NRG oncology/RTOG1205: a randomized phase II trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J Clin Oncol 41:1285
van Linde ME, Brahm CG, de Witt Hamer PC et al (2017) Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neurooncol 135(1):183–192
Ciammella P, Podgornii A, Galeandro M et al (2013) Hypofractionated stereotactic radiation therapy for recurrent glioblastoma: single institutional experience. Radiat Oncol 25(8):222
Socha J, Kepka L, Ghosh S et al (2016) Outcome of treatment of recurrent glioblastoma multiforme in elderly and/or frail patients. J Neurooncol 126(3):493–498
Kim HR, Kim KH, Kong DS et al (2015) Outcome of salvage treatment for recurrent glioblastoma. J Clin Neurosci 22(3):468–473
Bovi JA, Prah MA, Retzlaff AA et al (2020) Pulsed reduced dose rate radiotherapy in conjunction with bevacizumab or bevacizumab alone in recurrent high-grade glioma: survival outcomes. Int J Radiat Oncol Biol Phys 108(4):979–986
Bergman D, Modh A, Schultz L et al (2020) Randomized prospective trial of fractionated stereotactic radiosurgery with chemotherapy versus chemotherapy alone for bevacizumab-resistant high-grade glioma. J Neurooncol 148(2):353–361
Yasuda T, Muragaki Y, Nitta M et al (2018) Effectiveness of stereotactic radiotherapy and bevacizumab for recurrent high-grade gliomas: a potential therapy for isocitrate dehydrogenase wild-type recurrent high-grade gliomas. World Neurosurg 114:e1138–e1146
Schnell O, Thorsteinsdottir J, Fleischmann DF et al (2016) Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol 130(3):591–599
Yazici G, Cengiz M, Ozyigit G et al (2014) Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J Neurooncol 120(1):117–123
Wick W, Fricke H, Junge K et al (2014) A phase II, randomized, study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res 20(24):6304–6313
Miwa K, Matsuo M, Ogawa S et al (2014) Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol 14(9):181
Lovo EE, Moreira A, Barahona KC et al (2021) Stereotactic radiosurgery for recurrent glioblastoma multiforme: a retrospective multi-institutional experience. Cureus 13(10):e18480
Baehr A, Trog D, Oertel M et al (2020) Re-irradiation for recurrent glioblastoma multiforme: a critical comparison of different concepts. Strahlenther Onkol 196(5):457–464
Hasan S, Chen E, Lanciano R et al (2015) Salvage fractionated stereotactic radiotherapy with or without chemotherapy and immunotherapy for recurrent glioblastoma multiforme: a single institution experience. Front Oncol 15(5):106
Conti A, Pontoriero A, Arpa D et al (2012) Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir (Wien) 154(2):203–209
Fogh SE, Andrews DW, Glass J et al (2010) Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 28(18):3048–3053
Eberle F, Lautenschläger S, Engenhart-Cabillic R et al (2020) carbon ion beam reirradiation in recurrent high-grade glioma. Cancer Manag Res 28(12):633–639
Saeed AM, Khairnar R, Sharma AM et al (2020) Clinical outcomes in patients with recurrent glioblastoma treated with proton beam therapy reirradiation: analysis of the multi-institutional proton collaborative group registry. Adv Radiat Oncol 5(5):978–983
Scartoni D, Amelio D, Palumbo P et al (2020) Proton therapy re-irradiation preserves health-related quality of life in large recurrent glioblastoma. J Cancer Res Clin Oncol 146(6):1615–1622
Shen CJ, Kummerlowe MN, Redmond KJ et al (2018) Re-irradiation for malignant glioma: toward patient selection and defining treatment parameters for salvage. Adv Radiat Oncol 3(4):582–590
Cheon YJ, Jung TY, Jung S et al (2018) Efficacy of gamma knife radiosurgery for recurrent high-grade gliomas with limited tumor volume. J Korean Neurosurg Soc 61(4):516–524
Park KJ, Kano H, Iyer A et al (2012) Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol 107(2):323–333
Chan J, Jayamanne D, Wheeler H et al (2020) The role of large volume re-irradiation with Bevacizumab in chemorefractory high grade glioma. Clin Transl Radiat Oncol 9(22):33–39
Hundsberger T, Brügge D, Putora PM, Weder P, Weber J, Plasswilm L (2013) Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J Neurooncol 112(1):133–139
Guan Y, Xiong J, Pan M et al (2021) Safety and efficacy of hypofractionated stereotactic radiosurgery for high-grade gliomas at first recurrence: a single-center experience. BMC Cancer 21(1):123
Cuneo KC, Vredenburgh JJ, Sampson JH et al (2012) Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 82(5):2018–2024
Youland RS, Lee JY, Kreofsky CR et al (2018) Modern reirradiation for recurrent gliomas can safely delay tumor progression. Neurooncol Pract 5(1):46–55
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722. https://doi.org/10.1056/NEJMoa1308345
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708. https://doi.org/10.1056/NEJMoa1308573
Levin VA, Bidaut L, Hou P et al (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79(5):1487–1495
Sminia P, Mayer R (2012) External beam radiotherapy of recurrent glioma: radiation tolerance of the human brain. Cancers (Basel) 4:379–399
Lasocki A, Gaillard F (2019) Non-contrast-enhancing tumor: a new frontier in glioblastoma research. AJNR Am J Neuroradiol 40(5):758–765
Bhandari A, Marwah R, Smith J et al (2022) Machine learning imaging applications in the differentiation of true tumour progression from treatment-related effects in brain tumours: a systematic review and meta-analysis. J Med Imaging Radiat Oncol 66(6):781–797
Acknowledgements
This work has been accepted for presentation at the American Society for Radiation Oncology Annual Meeting 2023 in abstract form.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
RM conducted the search, collected the data, conducted the meta-analysis, completed the risk of bias assessment, assessed certainty of evidence, and wrote the manuscript. DX authored the study protocol, double-checked the search, data collection, meta-analysis and risk of bias assessment, and assisted in drafting the manuscript. TS, YYS, HG and SPN authored the study protocol and assisted in drafting the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marwah, R., Xing, D., Squire, T. et al. Reirradiation versus systemic therapy versus combination therapy for recurrent high-grade glioma: a systematic review and meta-analysis of survival and toxicity. J Neurooncol 164, 505–524 (2023). https://doi.org/10.1007/s11060-023-04441-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04441-0