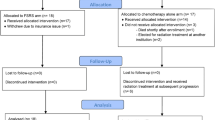
Abstract
Purpose
Recurrent glioblastoma is universally fatal with limited effective treatment options. The aim of this phase 2 study of Border Zone SRS plus bevacizumab was to evaluate OS in patients with recurrent GBM.
Methods
Patients with histologically confirmed GBM with recurrent disease who had received prior first-line treatment with fractionated radiotherapy and chemotherapy and eligible for SRS were enrolled. Bevacizumab 10 mg/kg was given day -1, day 14, and then every 14 days until disease progression. 1–14 days before BZ-SRS procedure, patients underwent brain MRI /MRS. MRS with measurement of choline-to-N-acetyl aspartate index (CNI) area ≥ 3 was targeted for SRS.
Results
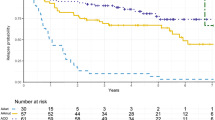
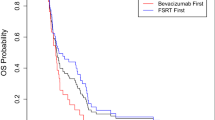
From 2015–2017, sixteen of planned 40 patients were enrolled. The median age was 62 (range, 48–74Y). 3/16 (0.188) participants experienced grade 2 toxicity. No AREs were reported. The mOS was 11.73 months compared to 8.74 months (P = 0.324) from date of SRS for the BZ-SRS and institutional historical controls, respectively. PFS-6 and OS-6 were 31.2% (p = 0.00294) and 81.2%(p = 0.058), respectively. Of 13 evaluable for best response: 1 CR (p = 0.077), 4 PR (p = 0.308), 7 SD (p = 0.538), and 1 PD (p = 0.077). 11/16 participants had MRS scans with an estimated probability that MRS changes a treatment plan of 0 (0, 0.285).
Conclusion
BZ-SRS with bevacizumab was feasible and well tolerated. There is no significant survival benefit using BZ-SRS with bevacizumab compared to institutional historical controls. Secondary analysis revealed a trend toward improved PFS-6, but not OS-6 after BZ-SRS. MRS scans did not result in changes to SRS treatment plans.


Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. The experimental data and results that support the findings of this study are available at https://www.clinicaltrials.gov/study/NCT02120287.
References
Mahaley MS Jr, Mettlin C, Natarajan N, Laws ER Jr, Peace BB (1989) National survey of patterns of care for brain-tumor patients. J Neurosurg 71:826–836. https://doi.org/10.3171/jns.1989.71.6.0826
Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D (2005) Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol 23:8863–8869. https://doi.org/10.1200/jco.2005.03.4157
Tsien CI, Pugh SL, Dicker AP, Raizer JJ, Matuszak MM, Lallana EC, Huang J, Algan O, Deb N, Portelance L, Villano JL, Hamm JT, Oh KS, Ali AN, Kim MM, Lindhorst SM, Mehta MP (2023) NRG Oncology/RTOG1205: a randomized phase II trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J Clin Oncol 41:1285–1295. https://doi.org/10.1200/jco.22.00164
Wu A (1992) Physics and dosimetry of the gamma knife. Neurosurg Clin N Am 3:35–50
Niranjan A, Monaco EA III, Kano H, Flickinger JC, Lunsford LD (2018) Stereotactic radiosurgery in the multimodality management of residual or recurrent glioblastoma multiforme. Prog Neurol Surg 31:48–61. https://doi.org/10.1159/000466998
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys 47:291–298. https://doi.org/10.1016/s0360-3016(99)00507-6
Bauman GS, Sneed PK, Wara WM, Stalpers LJ, Chang SM, McDermott MW, Gutin PH, Larson DA (1996) Reirradiation of primary CNS tumors. Int J Radiat Oncol Biol Phys 36:433–441. https://doi.org/10.1016/s0360-3016(96)00315-x
Sminia P, Mayer R (2012) External beam radiotherapy of recurrent glioma: radiation tolerance of the human brain. Cancers (Basel) 4:379–399. https://doi.org/10.3390/cancers4020379
Niranjan A, Kano H, Iyer A, Kondziolka D, Flickinger JC, Lunsford LD (2015) Role of adjuvant or salvage radiosurgery in the management of unresected residual or progressive glioblastoma multiforme in the pre-bevacizumab era. J Neurosurg 122:757–765. https://doi.org/10.3171/2014.11.Jns13295
Hochberg FH, Pruitt A (1980) Assumptions in the radiotherapy of glioblastoma. Neurology 30:907–911. https://doi.org/10.1212/wnl.30.9.907
Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG (1989) Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 16:1405–1409. https://doi.org/10.1016/0360-3016(89)90941-3
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66:865–874. https://doi.org/10.3171/jns.1987.66.6.0865
Beliën AT, Paganetti PA, Schwab ME (1999) Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter. J Cell Biol 144:373–384. https://doi.org/10.1083/jcb.144.2.373
Giese A, Kluwe L, Laube B, Meissner H, Berens ME, Westphal M (1996) Migration of human glioma cells on myelin. Neurosurgery 38:755–764
Demuth T, Berens ME (2004) Molecular mechanisms of glioma cell migration and invasion. J Neurooncol 70:217–228. https://doi.org/10.1007/s11060-004-2751-6
Pedersen PH, Edvardsen K, Garcia-Cabrera I, Mahesparan R, Thorsen J, Mathisen B, Rosenblum ML, Bjerkvig R (1995) Migratory patterns of lac-z transfected human glioma cells in the rat brain. Int J Cancer 62:767–771. https://doi.org/10.1002/ijc.2910620620
Gondi V, Pugh S, Tsien C, Chenevert T, Gilbert M, Omuro A, McDonough J, Aldape K, Srinivasan A, Rogers CL, Shi W, Suh JH, Algan O, Nedzi LA, Chan MD, Bahary JP, Mehta MP (2020) Radiotherapy (RT) Dose-intensification (DI) Using Intensity-modulated RT (IMRT) versus Standard-dose (SD) RT with Temozolomide (TMZ) in Newly Diagnosed Glioblastoma (GBM): Preliminary Results of NRG Oncology BN001. Int J Radiat Oncol Biolo Phys 108:22–23. https://doi.org/10.1016/j.ijrobp.2020.07.2109
Souhami L, Seiferheld W, Brachman D, Podgorsak EB, Werner-Wasik M, Lustig R, Schultz CJ, Sause W, Okunieff P, Buckner J, Zamorano L, Mehta MP, Curran WJ Jr (2004) Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of radiation therapy oncology group 93–05 protocol. Int J Radiat Oncol Biol Phys 60:853–860. https://doi.org/10.1016/j.ijrobp.2004.04.011
Kulinich DP, Sheppard JP, Nguyen T, Kondajji AM, Unterberger A, Duong C, Enomoto A, Patel K, Yang I (2021) Radiotherapy versus combination radiotherapy-bevacizumab for the treatment of recurrent high-grade glioma: a systematic review. Acta Neurochir (Wien) 163:1921–1934. https://doi.org/10.1007/s00701-021-04794-3
Chamberlain MC, Barba D, Kormanik P, Shea WM (1994) Stereotactic radiosurgery for recurrent gliomas. Cancer 74:1342–1347. https://doi.org/10.1002/1097-0142(19940815)74:4%3c1342::aid-cncr2820740426%3e3.0.co;2-y
Shrieve DC, Alexander E 3rd, Wen PY, Fine HA, Kooy HM, Black PM, Loeffler JS (1995) Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery 36:275–282. https://doi.org/10.1227/00006123-199502000-00006
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. https://doi.org/10.1200/jco.2008.19.8721
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729. https://doi.org/10.1200/jco.2007.12.2440
Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578. https://doi.org/10.1200/jco.1999.17.8.2572
Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB (1977) Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg 47:329–335. https://doi.org/10.3171/jns.1977.47.3.0329
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280. https://doi.org/10.1200/jco.1990.8.7.1277
Prados MD, Lamborn K, Yung WK, Jaeckle K, Robins HI, Mehta M, Fine HA, Wen PY, Cloughesy T, Chang S, Nicholas MK, Schiff D, Greenberg H, Junck L, Fink K, Hess K, Kuhn J (2006) A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American brain tumor consortium study. Neuro Oncol 8:189–193. https://doi.org/10.1215/15228517-2005-010
Buckner JC (2003) Factors influencing survival in high-grade gliomas. Semin Oncol 30:10–14. https://doi.org/10.1053/j.seminoncol.2003.11.031
Imbens G (2004) Nonparametric estimation of average treatment effects under exogeneity: a review. Rev Econ Stat 86(1):4–29
Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR (1999) Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res 59:3374–3378
Plate KH, Scholz A, Dumont DJ (2012) Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol 124:763–775. https://doi.org/10.1007/s00401-012-1066-5
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622. https://doi.org/10.1038/nrn2175
Branco-Price C, Evans CE, Johnson RS (2013) Endothelial hypoxic metabolism in carcinogenesis and dissemination: HIF-A isoforms are a NO metastatic phenomenon. Oncotarget 4:2567–2576. https://doi.org/10.18632/oncotarget.1461
Lund EL, Høg A, Olsen MW, Hansen LT, Engelholm SA, Kristjansen PE (2004) Differential regulation of VEGF, HIF1alpha and angiopoietin-1, -2 and -4 by hypoxia and ionizing radiation in human glioblastoma. Int J Cancer 108:833–838. https://doi.org/10.1002/ijc.11662
McGee MC, Hamner JB, Williams RF, Rosati SF, Sims TL, Ng CY, Gaber MW, Calabrese C, Wu J, Nathwani AC, Duntsch C, Merchant TE, Davidoff AM (2010) Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys 76:1537–1545. https://doi.org/10.1016/j.ijrobp.2009.12.010
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79:1487–1495. https://doi.org/10.1016/j.ijrobp.2009.12.061
Matuschek C, Bölke E, Nawatny J, Hoffmann TK, Peiper M, Orth K, Gerber PA, Rusnak E, Lammering G, Budach W (2011) Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol 187:135–139. https://doi.org/10.1007/s00066-010-2184-4
Furuse M, Kawabata S, Kuroiwa T, Miyatake S (2011) Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neurooncol 102:471–475. https://doi.org/10.1007/s11060-010-0333-3
Jiang X, Engelbach JA, Yuan L, Cates J, Gao F, Drzymala RE, Hallahan DE, Rich KM, Schmidt RE, Ackerman JJ, Garbow JR (2014) Anti-VEGF antibodies mitigate the development of radiation necrosis in mouse brain. Clin Cancer Res 20:2695–2702. https://doi.org/10.1158/1078-0432.Ccr-13-1941
Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, Lymberis S, Yamada Y, Chang J, Abrey LE (2009) Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 75:156–163. https://doi.org/10.1016/j.ijrobp.2008.10.043
Cuneo KC, Vredenburgh JJ, Sampson JH, Reardon DA, Desjardins A, Peters KB, Friedman HS, Willett CG, Kirkpatrick JP (2012) Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 82:2018–2024. https://doi.org/10.1016/j.ijrobp.2010.12.074
Park KJ, Kano H, Iyer A, Liu X, Niranjan A, Flickinger JC, Lieberman FS, Lunsford LD, Kondziolka D (2012) Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol 107:323–333. https://doi.org/10.1007/s11060-011-0744-9
Deibert CP, Ahluwalia MS, Sheehan JP, Link MJ, Hasegawa T, Yomo S, Feng WH, Li P, Flickinger JC, Lunsford LD, Kondziolka D (2013) Bevacizumab for refractory adverse radiation effects after stereotactic radiosurgery. J Neurooncol 115:217–223. https://doi.org/10.1007/s11060-013-1214-3
Abbassy M, Missios S, Barnett GH, Brewer C, Peereboom DM, Ahluwalia M, Neyman G, Chao ST, Suh JH, Vogelbaum MA (2018) Phase I trial of radiosurgery dose escalation plus bevacizumab in patients with recurrent/progressive glioblastoma. Neurosurgery 83:385–392. https://doi.org/10.1093/neuros/nyx369
Lovo EE, Moreira A, Barahona KC, Ramirez J, Campos F, Tobar C, Caceros V, Sallabanda M, Sallabanda K (2021) Stereotactic radiosurgery for recurrent glioblastoma multiforme: a retrospective multi-institutional experience. Cureus 13:e18480. https://doi.org/10.7759/cureus.18480
Weller M, Cloughesy T, Perry JR, Wick W (2013) Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro Oncol 15:4–27. https://doi.org/10.1093/neuonc/nos273
Einstein DB, Wessels B, Bangert B, Fu P, Nelson AD, Cohen M, Sagar S, Lewin J, Sloan A, Zheng Y, Williams J, Colussi V, Vinkler R, Maciunas R (2012) Phase II trial of radiosurgery to magnetic resonance spectroscopy-defined high-risk tumor volumes in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys 84:668–674. https://doi.org/10.1016/j.ijrobp.2012.01.020
Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH (2012) Radiation necrosis following treatment of high grade glioma–a review of the literature and current understanding. Acta Neurochir (Wien) 154:191–201. https://doi.org/10.1007/s00701-011-1228-6
Zhang H, Ma L, Wang Q, Zheng X, Wu C, Xu BN (2014) Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: a systematic review and meta-analysis. Eur J Radiol 83:2181–2189. https://doi.org/10.1016/j.ejrad.2014.09.018
van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A (2017) Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol 27:4129–4144. https://doi.org/10.1007/s00330-017-4789-9
Funding
This clinical trial was funded by a grant to Dr. Ajay Niranjan from Genentech and International Radiosurgery Research Foundation. This research received no other specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
Study design: AN, DM, JD, DL. Acquisition of data: AN, FSL, JD, DL. Statistical analysis: DM. Interpretation of the data: MM, AN, DM, JD, DL, CH. Drafting of the initial manuscript: MM, AN, DM, JD, DL, CH. Review and revision of the manuscript: all authors. Approval of the final manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
This clinical trial was funded by a grant to Dr. Ajay Niranjan from Genentech and International Radiosurgery Research Foundation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mantica, M., Drappatz, J., Lieberman, F. et al. Phase II study of border zone stereotactic radiosurgery with bevacizumab in patients with recurrent or progressive glioblastoma multiforme. J Neurooncol 164, 179–190 (2023). https://doi.org/10.1007/s11060-023-04398-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04398-0