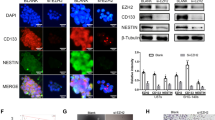
Abstract
Objective
Recent studies have increasingly shown that glioma stem cells (GSCs) are extremely important for developing and treating glioblastoma multiforme (GBM). The Broad-complex, Tram-track, and Bric-a-brac protein family is functionally related to a variety of tumor stem cells, and the role of SPOPL as a member of this family in GSCs deserves to be investigated.
Methods
To investigate the expression of SPOPL in GSCs and its impact on the prognosis of GBM patients by using clinical specimens, patient-derived primary GSCs and public databases. In vivo and in vitro, the effect of SPOPL on the proliferation, self-renewal, and differentiation ability of GSCs was explored. Probing the mechanism by which SPOPL affects the biological function of GSCs using RNA sequencing (RNA-seq) and rescue experiments.
Results
The expression of SPOPL was significantly upregulated in GSCs and GBM, and patients with high SPOPL expression had a poorer prognosis. SPOPL enhanced the proliferation and self-renewal ability of GSCs and enhanced the tumorigenicity of GSCs. The Notch signaling pathway was significantly inhibited in SPOPL knockdown GSCs. Activation or inhibition of the Notch signaling pathway rescued changes in the biological function of GSCs caused by altered SPOPL expression.
Conclusion
SPOPL can be used as a potential prognostic biomarker for GBM in clinical work and promotes the proliferation and stemness of GSCs by activating the Notch signaling pathway, which may be a potential molecule for targeting GSCs to treat GBM.





Similar content being viewed by others
References
Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, Toms S et al (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma a randomized clinical trial. Jama-J Am Med Assoc 318:2306–2316. https://doi.org/10.1001/jama.2017.18718
Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng SY et al (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. https://doi.org/10.1016/j.cell.2013.09.034
Barrette AM, Bouhaddou M, Birtwistle MR (2018) Integrating transcriptomic data with mechanistic systems pharmacology models for virtual drug combination trials. ACS Chem Neurosci 9:118–129. https://doi.org/10.1021/acschemneuro.7b00197
Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR et al (2019) An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178:835. https://doi.org/10.1016/j.cell.2019.06.024
Gimple RC, Bhargava S, Dixit D, Rich JN (2019) Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev 33:591–609. https://doi.org/10.1101/gad.324301.119
Sharifzad F, Ghavami S, Verdi J, Mardpour S, Sisakht MM, Azizi Z, Taghikhani A, Los MJ, Fakharian E, Ebrahimi M, Hamidieh AA (2019) Glioblastoma cancer stem cell biology: potential theranostic targets. Drug Resist Updates 42:35–45. https://doi.org/10.1016/j.drup.2018.03.003
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401. https://doi.org/10.1038/nature03128
Lee J, Kotliarova S, Kotliarov Y, Li AG, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9:391–403. https://doi.org/10.1016/j.ccr.2006.03.030
Chen J, Li YJ, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488:522. https://doi.org/10.1038/nature11287
Choo K-B, Chuang T-J, Lin W-Y, Chang C-M, Tsai Y-H, Huang C-J (2010) Evolutionary expansion of SPOP and associated TD/POZ gene family: impact of evolutionary route on gene expression pattern. Gene 460:39–47. https://doi.org/10.1016/j.gene.2010.04.003
Wang X, Jin J, Wan F, Zhao L, Chu H, Chen C, Liao G et al (2019) AMPK promotes SPOP-mediated NANOG degradation to regulate prostate cancer cell stemness. Dev Cell 48:345. https://doi.org/10.1016/j.devcel.2018.11.033
Tan P, Xu Y, Du Y, Wu L, Guo B, Huang S, Zhu J, Li B, Lin F, Yao L (2019) SPOP suppresses pancreatic cancer progression by promoting the degradation of NANOG. Cell Death Dis 10:794. https://doi.org/10.1038/s41419-019-2017-z
Zhang J, Wang S, Bai Y, Ali AM, Deng J, Chen Y, Fu Y, He M (2023) Mir-197-3p promotes osteosarcoma stemness and chemoresistance by inhibiting SPOPL. J Clin Med 12:1177. https://doi.org/10.3390/jcm12031177
Bao SD, Wu QL, McLendon RE, Hao YL, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760. https://doi.org/10.1038/nature05236
Zhang JY, Wang SB, Bai Y, Ali AM, Deng JW, Chen YS, Fu YH, He M (2023) Mir-197-3p promotes osteosarcoma stemness and chemoresistance by inhibiting SPOPL. J Clin Med. https://doi.org/10.3390/jcm12031177
Tan P, Xu YK, Du YC, Wu LL, Guo B, Huang SY, Zhu JH, Li B, Lin FJ, Yao L (2019) SPOP suppresses pancreatic cancer progression by promoting the degradation of NANOG. Cell Death Dis. https://doi.org/10.1038/s41419-019-2017-z
Errington WJ, Khan MQ, Bueler SA, Rubinstein JL, Chakrabartty A, Prive GG (2012) Adaptor protein self-assembly drives the control of a cullin-ring ubiquitin ligase. Structure 20:1141–1153. https://doi.org/10.1016/j.str.2012.04.009
Cai HC, Liu AM (2017) Spop regulates Gli3 activity and shh signaling in dorsoventral patterning of the mouse spinal cord. Dev Biol 432:72–85. https://doi.org/10.1016/j.ydbio.2017.04.002
Cai HC, Liu AM (2016) Spop promotes skeletal development and homeostasis by positively regulating ihh signaling. Proc Natl Acad Sci USA 113:14751–14756. https://doi.org/10.1073/pnas.1612520114
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445:111–115. https://doi.org/10.1038/nature05384
Mondal S, Bhattacharya K, Mandal C (2018) Nutritional stress reprograms dedifferention in glioblastoma multiforme driven by PTEN/Wnt/Hedgehog axis: a stochastic model of cancer stem cells. Cell Death Discov 4:110. https://doi.org/10.1038/s41420-018-0126-6
Baumann M, Krause M, Hill R (2008) Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 8:545–554. https://doi.org/10.1038/nrc2419
Karandish F, Froberg J, Borowicz P, Wilkinson JC, Choi Y, Mallik S (2018) Peptide-targeted, stimuli-responsive polymersomes for delivering a cancer stemness inhibitor to cancer stem cell microtumors. Colloids Surf B Biointerfaces 163:225–235. https://doi.org/10.1016/j.colsurfb.2017.12.036
Cheng Q, Zheng H, Li M, Wang HY, Guo XX, Zheng ZB, Chen CY, Liu JM, Zhan TC, Li ZW, Wu H, Han JD, Liu L, Tang TS, Chen Q, Du L (2022) LGR4 cooperates with PrPc to endow the stemness of colorectal cancer stem cells contributing to tumorigenesis and liver metastasis. Cancer Lett. https://doi.org/10.1016/j.canlet.2022.215725
Henkin RI (2019) Clinical and therapeutic implications of cancer stem cells. N Engl J Med 381:E19–E19
Wu XJ, Xiao SH, Zhang ML, Yang LX, Zhong J, Li B, Li FY, Xia X, Li XX, Zhou HK, Liu DW, Huang NU, Yang XS, Xiao FZ, Zhang N (2021) A novel protein encoded by circular SMO RNA is essential for hedgehog signaling activation and glioblastoma tumorigenicity. Genome Biol. https://doi.org/10.1186/s13059-020-02250-6
Gao XY, Xia X, Li FY, Zhang ML, Zhou HK, Wu XJ, Zhong J, Zhao Z, Zhao K, Liu DW, Xiao FZ, Xu Q, Jiang T, Li B, Cheng SY, Zhang N (2021) Circular RNA-encoded oncogenic E-cadherin variant promotes glioblastoma tumorigenicity through activation of EGFR-STAT3 signalling. Nat Cell Biol 23:278–. https://doi.org/10.1038/s41556-021-00639-4
Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, Morgan YG, Lok J, Grech A, Jones G, Khwaja A, Friis L, McMullin MF, Hunter A, Clark RE, Grimwade D (2015) Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol 16:1295–1305. https://doi.org/10.1016/s1470-2045(15)00193-x
Goranci-Buzhala G, Mariappan A, Ricci-Vitiani L, Josipovic N, Pacioni S, Gottardo M, Ptok J, Schaal H, Callaini G, Rajalingam K, Dynlacht B, Hadian K, Papantonis A, Pallini R, Gopalakrishnan J (2021) Cilium induction triggers differentiation of glioma stem cells. Cell Rep 36:109656. https://doi.org/10.1016/j.celrep.2021.109656
Li MX, Song D, Chen XY, Wang XZ, Xu LB, Yang M, Yang JY, Kalvakolanu DV, Wei XD, Liu XR, Li Y, Guo BF, Zhang L (2022) RSL3 triggers glioma stem cell differentiation via the Tgm2/AKT/ID1 signaling axis. Biochim Biophys Acta-Mol Basis Dis. https://doi.org/10.1016/j.bbadis.2022.166529
Stockhausen MT, Kristoffersen K, Poulsen HS (2010) The functional role of Notch signaling in human gliomas. Neurooncology 12:199–211. https://doi.org/10.1093/neuonc/nop022
Saito N, Aoki K, Hirai N, Fujita S, Iwama J, Ikota M, Nakayama H, Hayashi M, Ito K, Sakurai T, Iwabuchi S (2017) Notch pathway activation predicts resistance to bevacizumab therapy in glioblastoma. Cancer Res. https://doi.org/10.1158/1538-7445.Am2017-774
Sarkar S, Mirzaei R, Zemp FJ, Wei W, Senger DL, Robbins SM, Yong VW (2017) Activation of NOTCH signaling by tenascin-C promotes growth of human brain tumor-initiating cells. Cancer Res 77:3231–3243. https://doi.org/10.1158/0008-5472.Can-16-2171
Ojha R, Tantray I, Rimal S, Mitra S, Cheshier S, Lu BW (2022) Regulation of reverse electron transfer at mitochondrial complex I by unconventional Notch action in cancer stem cells. Dev Cell 57:260. https://doi.org/10.1016/j.devcel.2021.12.020
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81971663) and the Science and Technology Program Key Project of Guangzhou (Grant No. 201604020004).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experimental design and supervision of the article were performed by ZX. The experiments, data analysis, and the first draft of the manuscript were performed by TH, the collection of materials, part of the experiments were performed by RX, the bioinformatics analysis was performed by EH, and part of the animal experiments were performed by CL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University and conducted by the principles of the Declaration of Helsinki (No. 2020322). The Sun Yat-Sen University’s Institutional Animal Care and Use Committee approved all mice experiments (No. 2021173).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, T., Xuan, R., Han, E. et al. SPOPL induces tumorigenicity and stemness in glioma stem cells by activating Notch signaling. J Neurooncol 164, 157–170 (2023). https://doi.org/10.1007/s11060-023-04394-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04394-4