Abstract
Introduction
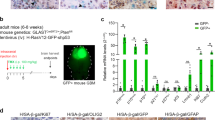
Glioma is the most common primary brain tumor and is often associated with treatment resistance and poor prognosis. Standard treatment typically involves radiotherapy and temozolomide-based chemotherapy, both of which induce cellular senescence—a tumor suppression mechanism.
Discussion
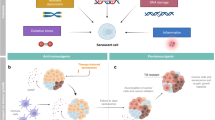
Gliomas employ various mechanisms to bypass or escape senescence and remain in a proliferative state. Importantly, senescent cells remain viable and secrete a large number of factors collectively known as the senescence-associated secretory phenotype (SASP) that, paradoxically, also have pro-tumorigenic effects. Furthermore, senescent cells may represent one form of tumor dormancy and play a role in glioma recurrence and progression.
Conclusion
In this article, we delineate an overview of senescence in the context of gliomas, including the mechanisms that lead to senescence induction, bypass, and escape. Furthermore, we examine the role of senescent cells in the tumor microenvironment and their role in tumor progression and recurrence. Additionally, we highlight potential therapeutic opportunities for targeting senescence in glioma.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621. https://doi.org/10.1016/0014-4827(61)90192-6
Di Leonardo A, Linke SP, Clarkin K, Wahl GM (1994) DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev 8:2540–2551. https://doi.org/10.1101/gad.8.21.2540
Krenning L, Feringa FM, Shaltiel IA et al (2014) Transient activation of p53 in G2 phase is sufficient to Induce Senescence. Mol Cell 55:59–72. https://doi.org/10.1016/j.molcel.2014.05.007
Gire V, Dulić V (2015) Senescence from G2 arrest, revisited. Cell Cycle 14:297–304. https://doi.org/10.1080/15384101.2014.1000134
McHugh D, Gil J (2018) Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol 217:65–77. https://doi.org/10.1083/jcb.201708092
Calcinotto A, Kohli J, Zagato E et al (2019) Cellular Senescence: aging, Cancer, and Injury. Physiol Rev 99:1047–1078. https://doi.org/10.1152/physrev.00020.2018
He S, Sharpless NE (2017) Senescence in Health and Disease. Cell 169:1000–1011. https://doi.org/10.1016/j.cell.2017.05.015
Parrinello S, Coppe J-P, Krtolica A, Campisi J (2005) Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci 118:485–496. https://doi.org/10.1242/jcs.01635
Hanahan D (2022) Hallmarks of Cancer: New Dimensions. Cancer Discov 12:31–46. https://doi.org/10.1158/2159-8290.CD-21-1059
Muñoz-Espín D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15:482–496. https://doi.org/10.1038/nrm3823
Campisi J (2013) Aging, cellular senescence, and cancer. Annu Rev Physiol 75:685–705. https://doi.org/10.1146/annurev-physiol-030212-183653
Foulkes I, Sharpless NE (2021) Cancer Grand Challenges: embarking on a new era of Discovery. Cancer Discov 11:23–27. https://doi.org/10.1158/2159-8290.CD-20-1657
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460. https://doi.org/10.1038/345458a0
Herbig U, Jobling WA, Chen BPC et al (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14:501–513. https://doi.org/10.1016/s1097-2765(04)00256-4
Serrano M, Lin AW, McCurrach ME et al (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593–602. https://doi.org/10.1016/s0092-8674(00)81902-9
Di Micco R, Fumagalli M, Cicalese A et al (2006) Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444:638–642. https://doi.org/10.1038/nature05327
Zhu J, Woods D, McMahon M, Bishop JM (1998) Senescence of human fibroblasts induced by oncogenic raf. Genes Dev 12:2997–3007
Chang B-D, Swift ME, Shen M et al (2002) Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc Natl Acad Sci U S A 99:389–394. https://doi.org/10.1073/pnas.012602599
Schmitt CA, Fridman JS, Yang M et al (2002) A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109:335–346. https://doi.org/10.1016/s0092-8674(02)00734-1
te Poele RH, Okorokov AL, Jardine L et al (2002) DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 62:1876–1883
Oh CW, Bump EA, Kim JS et al (2001) Induction of a senescence-like phenotype in bovine aortic endothelial cells by ionizing radiation. Radiat Res 156:232–240. https://doi.org/10.1667/0033-7587(2001)156[0232:ioaslp]2.0.co;2
Suzuki K, Mori I, Nakayama Y et al (2001) Radiation-induced senescence-like growth arrest requires TP53 function but not telomere shortening. Radiat Res 155:248–253. https://doi.org/10.1667/0033-7587(2001)155[0248:rislga]2.0.co;2
Childs BG, Baker DJ, Kirkland JL et al (2014) Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15:1139–1153. https://doi.org/10.15252/embr.201439245
Michaloglou C, Vredeveld LCW, Soengas MS et al (2005) BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436:720–724. https://doi.org/10.1038/nature03890
Alimonti A, Nardella C, Chen Z et al (2010) A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest 120:681–693. https://doi.org/10.1172/JCI40535
Anerillas C, Herman AB, Rossi M et al (2022) Early SRC activation skews cell fate from apoptosis to senescence. Sci Adv 8:eabm0756. https://doi.org/10.1126/sciadv.abm0756
Kumari R, Jat P (2021) Mechanisms of Cellular Senescence: cell cycle arrest and Senescence Associated Secretory phenotype. Front Cell Dev Biol 9
McConnell BB, Starborg M, Brookes S, Peters G (1998) Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol 8:351–354. https://doi.org/10.1016/S0960-9822(98)70137-X
Chen Q, Ames BN (1994) Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci U S A 91:4130–4134
Nishio K, Inoue A, Qiao S et al (2001) Senescence and cytoskeleton: overproduction of vimentin induces senescent-like morphology in human fibroblasts. Histochem Cell Biol 116:321–327. https://doi.org/10.1007/s004180100325
Walen KH (2006) Human diploid fibroblast cells in senescence; cycling through polyploidy to mitotic cells. In Vitro Cell Dev Biol Anim 42:216–224. https://doi.org/10.1290/0603019.1
Cho KA, Ryu SJ, Oh YS et al (2004) Morphological Adjustment of senescent cells by modulating Caveolin-1 Status*. J Biol Chem 279:42270–42278. https://doi.org/10.1074/jbc.M402352200
Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O (2009) Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4:1798–1806. https://doi.org/10.1038/nprot.2009.191
Lee BY, Han JA, Im JS et al (2006) Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 5:187–195. https://doi.org/10.1111/j.1474-9726.2006.00199.x
Shelton DN, Chang E, Whittier PS et al (1999) Microarray analysis of replicative senescence. Curr Biol CB 9:939–945. https://doi.org/10.1016/s0960-9822(99)80420-5
Wang L, Lankhorst L, Bernards R (2022) Exploiting senescence for the treatment of cancer. Nat Rev Cancer 22:340–355. https://doi.org/10.1038/s41568-022-00450-9
Coppé J-P, Patil CK, Rodier F et al (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6:2853–2868. https://doi.org/10.1371/journal.pbio.0060301
Schmitt CA, Wang B, Demaria M (2022) Senescence and cancer — role and therapeutic opportunities. Nat Rev Clin Oncol 19:619–636. https://doi.org/10.1038/s41571-022-00668-4
Fletcher-Sananikone E, Kanji S, Tomimatsu N et al (2021) Elimination of Radiation-Induced Senescence in the Brain Tumor Microenvironment attenuates Glioblastoma Recurrence. Cancer Res 81:5935–5947. https://doi.org/10.1158/0008-5472.CAN-21-0752
Beltzig L, Schwarzenbach C, Leukel P et al (2022) Senescence is the Main Trait Induced by Temozolomide in Glioblastoma cells. Cancers 14:2233. https://doi.org/10.3390/cancers14092233
Chatterjee D, Chakrabarti O (2022) Role of stress granules in modulating senescence and promoting cancer progression: special emphasis on glioma. Int J Cancer 150:551–561. https://doi.org/10.1002/ijc.33787
Weller M, Wick W, Aldape K et al (2015) Glioma. Nat Rev Dis Primer 1:15017. https://doi.org/10.1038/nrdp.2015.17
Ostrom QT, Cioffi G, Waite K et al (2021) CBTRUS Statistical Report: primary brain and other Central Nervous System Tumors diagnosed in the United States in 2014–2018. Neuro-Oncol 23:iii1–iii105. https://doi.org/10.1093/neuonc/noab200
Wesseling P, Kros JM, Jeuken JWM (2011) The pathological diagnosis of diffuse gliomas: towards a smart synthesis of microscopic and molecular information in a multidisciplinary context. Diagn Histopathol 17:486–494. https://doi.org/10.1016/j.mpdhp.2011.08.005
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Weller M, van den Bent M, Preusser M et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18:170–186. https://doi.org/10.1038/s41571-020-00447-z
Yang K, Wu Z, Zhang H et al (2022) Glioma targeted therapy: insight into future of molecular approaches. Mol Cancer 21:39. https://doi.org/10.1186/s12943-022-01513-z
Jakola AS, Skjulsvik AJ, Myrmel KS et al (2017) Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. https://doi.org/10.1093/annonc/mdx230
Liang J, Lv X, Lu C et al (2020) Prognostic factors of patients with gliomas – an analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer 20:35. https://doi.org/10.1186/s12885-019-6511-6
Chojak R, Koźba-Gosztyła M, Słychan K et al (2021) Impact of surgical resection of butterfly glioblastoma on survival: a meta-analysis based on comparative studies. Sci Rep 11:13934. https://doi.org/10.1038/s41598-021-93441-z
Mladenovsk M, Valkov I, Ovcharov M et al (2021) High Grade Glioma surgery—clinical aspects and prognosis. Folia Med (Plovdiv) 63:35–41. https://doi.org/10.3897/folmed.63.e55255
Nayak L, Reardon DA (2017) High-grade gliomas. Contin Minneap Minn 23:1548–1563. https://doi.org/10.1212/CON.0000000000000554
Claus EB, Walsh KM, Wiencke J et al (2015) Survival and low grade glioma: the emergence of genetic information. Neurosurg Focus 38:E6. https://doi.org/10.3171/2014.10.FOCUS12367
Loeffler JS, Alexander E, Hochberg FH et al (1990) Clinical patterns of failure following stereotactic interstitial irradiation for malignant gliomas. Int J Radiat Oncol Biol Phys 19:1455–1462. https://doi.org/10.1016/0360-3016(90)90358-q
Shaw EG, Berkey B, Coons SW et al (2008) Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg 109:835–841. https://doi.org/10.3171/JNS/2008/109/11/0835
Fukuya Y, Ikuta S, Maruyama T et al (2019) Tumor recurrence patterns after surgical resection of intracranial low-grade gliomas. J Neurooncol 144:519–528. https://doi.org/10.1007/s11060-019-03250-8
Santos-de-Frutos K, Djouder N (2021) When dormancy fuels tumour relapse. Commun Biol 4:747. https://doi.org/10.1038/s42003-021-02257-0
Stupp R, Taillibert S, Kanner AA et al (2015) Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide alone for Glioblastoma: a Randomized Clinical Trial. JAMA 314:2535–2543. https://doi.org/10.1001/jama.2015.16669
Stupp R, Taillibert S, Kanner A et al (2017) Effect of Tumor-Treating Fields Plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a Randomized Clinical Trial. JAMA 318:2306–2316. https://doi.org/10.1001/jama.2017.18718
Shay JW, Wright WE (2000) Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol 1:72–76. https://doi.org/10.1038/35036093
de Lange T (2009) How telomeres solve the end-protection problem. Science 326:948–952. https://doi.org/10.1126/science.1170633
Scully R, Panday A, Elango R, Willis NA (2019) DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Biol 20:698–714. https://doi.org/10.1038/s41580-019-0152-0
Hewitt G, Jurk D, Marques FDM et al (2012) Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 3:708. https://doi.org/10.1038/ncomms1708
Crouch J, Shvedova M, Thanapaul RJRS et al (2022) Epigenetic regulation of Cellular Senescence. https://doi.org/10.3390/cells11040672. Cells 11:
Nacarelli T, Liu P, Zhang R (2017) Epigenetic basis of Cellular Senescence and its implications in aging. Genes 8:343. https://doi.org/10.3390/genes8120343
Sakaki M, Ebihara Y, Okamura K et al (2017) Potential roles of DNA methylation in the initiation and establishment of replicative senescence revealed by array-based methylome and transcriptome analyses. PLoS ONE 12:e0171431. https://doi.org/10.1371/journal.pone.0171431
Bianchessi V, Vinci MC, Nigro P et al (2016) Methylation profiling by bisulfite sequencing analysis of the mtDNA non-coding region in replicative and senescent endothelial cells. Mitochondrion 27:40–47. https://doi.org/10.1016/j.mito.2016.02.004
Sidler C, Kovalchuk O, Kovalchuk I (2017) Epigenetic regulation of Cellular Senescence and Aging. Front Genet 8
Campisi J (1997) The biology of replicative senescence. Eur J Cancer 33:703–709. https://doi.org/10.1016/S0959-8049(96)00058-5
Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192:547–556. https://doi.org/10.1083/jcb.201009094
Kim S, Chowdhury T, Yu HJ et al (2022) The telomere maintenance mechanism spectrum and its dynamics in gliomas. Genome Med 14:88. https://doi.org/10.1186/s13073-022-01095-x
Cong Y-S, Wright WE, Shay JW (2002) Human telomerase and its regulation. Microbiol Mol Biol Rev 66:407–425. https://doi.org/10.1128/MMBR.66.3.407-425.2002
Artandi SE, DePinho RA (2010) Telomeres and telomerase in cancer. Carcinogenesis 31:9–18. https://doi.org/10.1093/carcin/bgp268
Killela PJ, Reitman ZJ, Jiao Y et al (2013) TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A 110:6021–6026. https://doi.org/10.1073/pnas.1303607110
Barger CJ, Suwala AK, Soczek KM et al (2022) Conserved features of TERT promoter duplications reveal an activation mechanism that mimics hotspot mutations in cancer. Nat Commun 13:5430. https://doi.org/10.1038/s41467-022-33099-x
Körber V, Yang J, Barah P et al (2019) Evolutionary trajectories of IDHWT Glioblastomas reveal a common path of early tumorigenesis instigated years ahead of initial diagnosis. Cancer Cell 35:692–704e12. https://doi.org/10.1016/j.ccell.2019.02.007
Terzi NK, Yilmaz I, Oz AB (2022) The place and prognostic value of TERT promoter mutation in molecular classification in Grade II-III glial tumors and primary glioblastomas. Turk Patoloji Derg 38:90–98. https://doi.org/10.5146/tjpath.2021.01555
Heidenreich B, Rachakonda SP, Hosen I et al (2015) TERT promoter mutations and telomere length in adult malignant gliomas and recurrences. Oncotarget 6:10617–10633. https://doi.org/10.18632/oncotarget.3329
Simon M, Hosen I, Gousias K et al (2015) TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro-Oncol 17:45–52. https://doi.org/10.1093/neuonc/nou158
Nonoguchi N, Ohta T, Oh J-E et al (2013) TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol (Berl) 126:931–937. https://doi.org/10.1007/s00401-013-1163-0
Spiegl-Kreinecker S, Lötsch D, Ghanim B et al (2015) Prognostic quality of activating TERT promoter mutations in glioblastoma: interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro-Oncol 17:1231–1240. https://doi.org/10.1093/neuonc/nov010
Yuan Y, Qi C, Maling G et al (2016) TERT mutation in glioma: frequency, prognosis and risk. J Clin Neurosci Off J Neurosurg Soc Australas 26:57–62. https://doi.org/10.1016/j.jocn.2015.05.066
Vuong HG, Altibi AMA, Duong UNP et al (2017) TERT promoter mutation and its interaction with IDH mutations in glioma: combined TERT promoter and IDH mutations stratifies lower-grade glioma into distinct survival subgroups-A meta-analysis of aggregate data. Crit Rev Oncol Hematol 120:1–9. https://doi.org/10.1016/j.critrevonc.2017.09.013
Arita H, Matsushita Y, Machida R et al (2020) TERT promoter mutation confers favorable prognosis regardless of 1p/19q status in adult diffuse gliomas with IDH1/2 mutations. Acta Neuropathol Commun 8:201. https://doi.org/10.1186/s40478-020-01078-2
Killela PJ, Pirozzi CJ, Healy P et al (2014) Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget 5:1515–1525. https://doi.org/10.18632/oncotarget.1765
Labussière M, Boisselier B, Mokhtari K et al (2014) Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 83:1200–1206. https://doi.org/10.1212/WNL.0000000000000814
Eckel-Passow JE, Lachance DH, Molinaro AM et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508. https://doi.org/10.1056/NEJMoa1407279
Vuong HG, Nguyen TQ, Ngo TNM et al (2020) The interaction between TERT promoter mutation and MGMT promoter methylation on overall survival of glioma patients: a meta-analysis. BMC Cancer 20:897. https://doi.org/10.1186/s12885-020-07364-5
Kim HS, Kwon MJ, Song JH et al (2018) Clinical implications of TERT promoter mutation on IDH mutation and MGMT promoter methylation in diffuse gliomas. Pathol—Res Pract 214:881–888. https://doi.org/10.1016/j.prp.2018.04.002
Hegi ME, Diserens A-C, Gorlia T et al (2005) MGMT Gene silencing and benefit from Temozolomide in Glioblastoma. N Engl J Med 352:997–1003. https://doi.org/10.1056/NEJMoa043331
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Weller M, Stupp R, Reifenberger G et al (2010) MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 6:39–51. https://doi.org/10.1038/nrneurol.2009.197
Giunco S, Padovan M, Angelini C et al (2023) Prognostic role and interaction of TERT promoter status, telomere length and MGMT promoter methylation in newly diagnosed IDH wild-type glioblastoma patients. ESMO Open 8:101570. https://doi.org/10.1016/j.esmoop.2023.101570
Diplas BH, He X, Brosnan-Cashman JA et al (2018) The genomic landscape of TERT promoter wildtype-IDH wildtype glioblastoma. Nat Commun 9:2087. https://doi.org/10.1038/s41467-018-04448-6
Dilley RL, Verma P, Cho NW et al (2016) Break-induced telomere synthesis underlies alternative telomere maintenance. Nature 539:54–58. https://doi.org/10.1038/nature20099
Clynes D, Jelinska C, Xella B et al (2015) Suppression of the alternative lengthening of telomere pathway by the chromatin remodelling factor ATRX. Nat Commun 6:7538. https://doi.org/10.1038/ncomms8538
Lewis PW, Elsaesser SJ, Noh K-M et al (2010) Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc Natl Acad Sci U S A 107:14075–14080. https://doi.org/10.1073/pnas.1008850107
Heaphy CM, de Wilde RF, Jiao Y et al (2011) Altered telomeres in tumors with ATRX and DAXX mutations. Science 333:425. https://doi.org/10.1126/science.1207313
Jiao Y, Killela PJ, Reitman ZJ et al (2012) Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 3:709–722
Osterwald S, Deeg KI, Chung I et al (2015) PML induces compaction, TRF2 depletion and DNA damage signaling at telomeres and promotes their alternative lengthening. J Cell Sci 128:1887–1900. https://doi.org/10.1242/jcs.148296
Grobelny JV, Godwin AK, Broccoli D (2000) ALT-associated PML bodies are present in viable cells and are enriched in cells in the G(2)/M phase of the cell cycle. J Cell Sci 113 Pt 24:4577–4585. https://doi.org/10.1242/jcs.113.24.4577
Yeager TR, Neumann AA, Englezou A et al (1999) Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res 59:4175–4179
Draskovic I, Arnoult N, Steiner V et al (2009) Probing PML body function in ALT cells reveals spatiotemporal requirements for telomere recombination. Proc Natl Acad Sci 106:15726–15731. https://doi.org/10.1073/pnas.0907689106
Loe TK, Li JSZ, Zhang Y et al (2020) Telomere length heterogeneity in ALT cells is maintained by PML-dependent localization of the BTR complex to telomeres. Genes Dev 34:650–662. https://doi.org/10.1101/gad.333963.119
Min J, Wright WE, Shay JW (2019) Clustered telomeres in phase-separated nuclear condensates engage mitotic DNA synthesis through BLM and RAD52. Genes Dev 33:814–827. https://doi.org/10.1101/gad.324905.119
Li F, Deng Z, Zhang L et al (2019) ATRX loss induces telomere dysfunction and necessitates induction of alternative lengthening of telomeres during human cell immortalization. EMBO J 38:e96659. https://doi.org/10.15252/embj.201796659
Kannan K, Inagaki A, Silber J et al (2012) Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget 3:1194–1203. https://doi.org/10.18632/oncotarget.689
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. https://doi.org/10.1056/NEJMoa0808710
Sanson M, Marie Y, Paris S et al (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol Off J Am Soc Clin Oncol 27:4150–4154. https://doi.org/10.1200/JCO.2009.21.9832
Hartmann C, Meyer J, Balss J et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol (Berl) 118:469–474. https://doi.org/10.1007/s00401-009-0561-9
Ferreira MSV, Sørensen MD, Pusch S et al (2020) Alternative lengthening of telomeres is the major telomere maintenance mechanism in astrocytoma with isocitrate dehydrogenase 1 mutation. J Neurooncol 147:1–14. https://doi.org/10.1007/s11060-020-03394-y
Minasi S, Baldi C, Gianno F et al (2021) Alternative lengthening of telomeres in molecular subgroups of paediatric high-grade glioma. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 37:809–818. https://doi.org/10.1007/s00381-020-04933-8
McDonald KL, McDonnell J, Muntoni A et al (2010) Presence of alternative lengthening of telomeres mechanism in patients with glioblastoma identifies a less aggressive tumor type with longer survival. J Neuropathol Exp Neurol 69:729–736. https://doi.org/10.1097/NEN.0b013e3181e576cf
Hakin-Smith V, Jellinek DA, Levy D et al (2003) Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet Lond Engl 361:836–838. https://doi.org/10.1016/s0140-6736(03)12681-5
Hu Y, Shi G, Zhang L et al (2016) Switch telomerase to ALT mechanism by inducing telomeric DNA damages and dysfunction of ATRX and DAXX. Sci Rep 6:32280. https://doi.org/10.1038/srep32280
Aquilanti E, Kageler L, Wen PY, Meyerson M (2021) Telomerase as a therapeutic target in glioblastoma. Neuro-Oncol 23:2004–2013. https://doi.org/10.1093/neuonc/noab203
Olympios N, Gilard V, Marguet F et al (2021) TERT promoter alterations in Glioblastoma: a systematic review. Cancers 13:1147. https://doi.org/10.3390/cancers13051147
Asai A, Oshima Y, Yamamoto Y et al (2003) A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res 63:3931–3939
Herbert B-S, Gellert GC, Hochreiter A et al (2005) Lipid modification of GRN163, an N3’P5’ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene 24:5262–5268. https://doi.org/10.1038/sj.onc.1208760
Marian CO, Cho SK, McEllin BM et al (2010) The Telomerase Antagonist Imetelstat efficiently targets Glioblastoma Tumor-Initiating cells leading to decreased proliferation and Tumor Growth. Clin Cancer Res Off J Am Assoc Cancer Res 16:154–163. https://doi.org/10.1158/1078-0432.CCR-09-2850
Ferrandon S, Malleval C, El Hamdani B et al (2015) Telomerase inhibition improves tumor response to radiotherapy in a murine orthotopic model of human glioblastoma. Mol Cancer 14:134. https://doi.org/10.1186/s12943-015-0376-3
Salloum R, Hummel TR, Kumar SS et al (2016) A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: a pediatric brain tumor consortium study. J Neurooncol 129:443–451. https://doi.org/10.1007/s11060-016-2189-7
You Y, Pu P, Huang Q et al (2006) Antisense telomerase RNA inhibits the growth of human glioma cells in vitro and in vivo. Int J Oncol 28:1225–1232
Kondo S, Kondo Y, Li G et al (1998) Targeted therapy of human malignant glioma in a mouse model by 2-5A antisense directed against telomerase RNA. Oncogene 16:3323–3330. https://doi.org/10.1038/sj.onc.1201885
Zhao P, Wang C, Fu Z et al (2007) Lentiviral vector mediated siRNA knock-down of hTERT results in diminished capacity in invasiveness and in vivo growth of human glioma cells in a telomere length-independent manner. Int J Oncol 31:361–368
Lavanya Ch, Sibin MK, Srinivas Bharath MM et al (2016) RNA interference mediated downregulation of human telomerase reverse transcriptase (hTERT) in LN18 cells. Cytotechnology 68:2311–2321. https://doi.org/10.1007/s10616-016-0025-8
Wang T, Xue Y, Wang M, Sun Q (2012) Silencing of the hTERT gene through RNA interference induces apoptosis via bax/bcl-2 in human glioma cells. Oncol Rep 28:1153–1158. https://doi.org/10.3892/or.2012.1952
Mender I, Gryaznov S, Dikmen ZG et al (2015) Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2’-deoxyguanosine. Cancer Discov 5:82–95. https://doi.org/10.1158/2159-8290.CD-14-0609
Yu S, Wei S, Savani M et al (2021) A modified nucleoside 6-thio-2’-deoxyguanosine exhibits anti-tumor activity in Gliomas. Clin Cancer Res Off J Am Assoc Cancer Res 27:6800–6814. https://doi.org/10.1158/1078-0432.CCR-21-0374
Zhang P, Rashidi A, Zhao J et al (2023) STING agonist-loaded, CD47/PD-L1-targeting nanoparticles potentiate antitumor immunity and radiotherapy for glioblastoma. Nat Commun 14:1610. https://doi.org/10.1038/s41467-023-37328-9
Reardon DA, Brem S, Desai AS et al (2020) INO-5401 and INO-9012 delivered intramuscularly (IM) with electroporation (EP) in combination with cemiplimab (REGN2810) in newly diagnosed glioblastoma (GBM): interim results. J Clin Oncol 38:2514–2514. https://doi.org/10.1200/JCO.2020.38.15_suppl.2514
Takahashi M, Miki S, Fujimoto K et al (2019) Eribulin penetrates brain tumor tissue and prolongs survival of mice harboring intracerebral glioblastoma xenografts. Cancer Sci 110:2247–2257. https://doi.org/10.1111/cas.14067
Miki S, Imamichi S, Fujimori H et al (2018) Concomitant administration of radiation with eribulin improves the survival of mice harboring intracerebral glioblastoma. Cancer Sci 109:2275–2285. https://doi.org/10.1111/cas.13637
Takahashi M, Kawashima S, Otake Y et al (2022) A phase II, multicenter, single-arm trial of eribulin in patients with bevacizumab-resistant recurrent glioblastoma. J Clin Oncol 40:2036–2036. https://doi.org/10.1200/JCO.2022.40.16_suppl.2036
Lagah S, Tan I-L, Radhakrishnan P et al (2014) RHPS4 G-Quadruplex ligand induces anti-proliferative Effects in Brain Tumor cells. PLoS ONE 9:e86187. https://doi.org/10.1371/journal.pone.0086187
Berardinelli F, Siteni S, Tanzarella C et al (2015) The G-quadruplex-stabilising agent RHPS4 induces telomeric dysfunction and enhances radiosensitivity in glioblastoma cells. DNA Repair 25:104–115. https://doi.org/10.1016/j.dnarep.2014.10.009
Berardinelli F, Tanori M, Muoio D et al (2019) G-quadruplex ligand RHPS4 radiosensitizes glioblastoma xenograft in vivo through a differential targeting of bulky differentiated- and stem-cancer cells. J Exp Clin Cancer Res 38:311. https://doi.org/10.1186/s13046-019-1293-x
Zhou G, Liu X, Li Y et al (2016) Telomere targeting with a novel G-quadruplex-interactive ligand BRACO-19 induces T-loop disassembly and telomerase displacement in human glioblastoma cells. Oncotarget 7:14925–14939. https://doi.org/10.18632/oncotarget.7483
Rossi A, Russo G, Puca A et al (2009) The antiretroviral nucleoside analogue Abacavir reduces cell growth and promotes differentiation of human medulloblastoma cells. Int J Cancer J Int Cancer 125:235–243. https://doi.org/10.1002/ijc.24331
Rivas S, Govindarajan V, Valdez MM et al (2022) Exth-34. Anti-retroviral repurposing for treatment of glioblastoma. Neuro-Oncol 24:vii216–vii217. https://doi.org/10.1093/neuonc/noac209.832
Lavanya C, Venkataswamy MM, Sibin MK et al (2018) Down regulation of human telomerase reverse transcriptase (hTERT) expression by BIBR1532 in human glioblastoma LN18 cells. Cytotechnology 70:1143–1154. https://doi.org/10.1007/s10616-018-0205-9
Andrade da Mota TH, Reis Guimarães AF, Silva de Carvalho A et al (2021) Effects of in vitro short- and long-term treatment with telomerase inhibitor in U-251 glioma cells. Tumour Biol J Int Soc Oncodevelopmental Biol Med 43:327–340. https://doi.org/10.3233/TUB-211515
Gurung RL, Lim HK, Venkatesan S et al (2014) Targeting DNA-PKcs and telomerase in brain tumour cells. Mol Cancer 13:232. https://doi.org/10.1186/1476-4598-13-232
Haase S, Garcia-Fabiani MB, Carney S et al (2018) Mutant ATRX: uncovering a new therapeutic target for glioma. Expert Opin Ther Targets 22:599–613. https://doi.org/10.1080/14728222.2018.1487953
Fan H-C, Chen C-M, Chi C-S et al (2019) Targeting telomerase and ATRX/DAXX inducing Tumor Senescence and apoptosis in the malignant glioma. Int J Mol Sci 20:200. https://doi.org/10.3390/ijms20010200
Zhang J-M, Zou L (2020) Alternative lengthening of telomeres: from molecular mechanisms to therapeutic outlooks. Cell Biosci 10:30. https://doi.org/10.1186/s13578-020-00391-6
de Lima MF, Freitas MO, Hamedani MK et al (2022) Consecutive inhibition of telomerase and alternative Lengthening Pathway promotes Hodgkin’s Lymphoma Cell Death. Biomedicines 10:2299. https://doi.org/10.3390/biomedicines10092299
Liu X-L, Ding J, Meng L-H (2018) Oncogene-induced senescence: a double edged sword in cancer. Acta Pharmacol Sin 39:1553–1558. https://doi.org/10.1038/aps.2017.198
Chandeck C, Mooi WJ (2010) Oncogene-induced Cellular Senescence. Adv Anat Pathol 17:42–48. https://doi.org/10.1097/PAP.0b013e3181c66f4e
Chen Z, Trotman LC, Shaffer D et al (2005) Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436:725–730. https://doi.org/10.1038/nature03918
Wu C-H, van Riggelen J, Yetil A et al (2007) Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci 104:13028–13033. https://doi.org/10.1073/pnas.0701953104
Astle MV, Hannan KM, Ng PY et al (2012) AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene 31:1949–1962. https://doi.org/10.1038/onc.2011.394
Zhu H, Blake S, Kusuma FK et al (2020) Oncogene-induced senescence: from biology to therapy. Mech Ageing Dev 187:111229. https://doi.org/10.1016/j.mad.2020.111229
Wan PTC, Garnett MJ, Roe SM et al (2004) Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116:855–867. https://doi.org/10.1016/s0092-8674(04)00215-6
Yaswen P, Campisi J (2007) Oncogene-Induced Senescence Pathways weave an intricate tapestry. Cell 128:233–234. https://doi.org/10.1016/j.cell.2007.01.005
Kowalewski A, Durślewicz J, Zdrenka M et al (2020) Clinical relevance of BRAF V600E Mutation Status in Brain Tumors with a focus on a Novel Management Algorithm. Target Oncol 15:531–540. https://doi.org/10.1007/s11523-020-00735-9
Raabe EH, Lim KS, Kim JM et al (2011) BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin Cancer Res Off J Am Assoc Cancer Res 17:3590–3599. https://doi.org/10.1158/1078-0432.CCR-10-3349
Schiffman JD, Hodgson JG, VandenBerg SR et al (2010) Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res 70:512–519. https://doi.org/10.1158/0008-5472.CAN-09-1851
Mistry M, Zhukova N, Merico D et al (2015) BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J Clin Oncol Off J Am Soc Clin Oncol 33:1015–1022. https://doi.org/10.1200/JCO.2014.58.3922
Bouchè V, Aldegheri G, Donofrio CA et al (2021) BRAF Signaling Inhibition in Glioblastoma: which clinical Perspectives? Front Oncol 11
Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273:13375–13378. https://doi.org/10.1074/jbc.273.22.13375
Maehama T, Dixon JE (1999) PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol 9:125–128. https://doi.org/10.1016/s0962-8924(99)01519-6
Georgescu M-M (2010) PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer 1:1170–1177. https://doi.org/10.1177/1947601911407325
Lee J-J, Kim BC, Park M-J et al (2011) PTEN status switches cell fate between premature senescence and apoptosis in glioma exposed to ionizing radiation. Cell Death Differ 18:666–677. https://doi.org/10.1038/cdd.2010.139
McLendon R, Friedman A, Bigner D et al (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068. https://doi.org/10.1038/nature07385
Verhaak RGW, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. https://doi.org/10.1016/j.ccr.2009.12.020
Smith JS, Tachibana I, Passe SM et al (2001) PTEN Mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and Glioblastoma Multiforme. JNCI J Natl Cancer Inst 93:1246–1256. https://doi.org/10.1093/jnci/93.16.1246
Han F, Hu R, Yang H et al (2016) PTEN gene mutations correlate to poor prognosis in glioma patients: a meta-analysis. OncoTargets Ther 9:3485–3492. https://doi.org/10.2147/OTT.S99942
Patel AP, Tirosh I, Trombetta JJ et al (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344:1396–1401. https://doi.org/10.1126/science.1254257
Tan C, Wei Y, Ding X et al (2022) Cell senescence-associated genes predict the malignant characteristics of glioblastoma. Cancer Cell Int 22:411. https://doi.org/10.1186/s12935-022-02834-1
Lauko A, Lo A, Ahluwalia MS, Lathia JD (2022) Cancer cell heterogeneity & plasticity in glioblastoma and brain tumors. Semin Cancer Biol 82:162–175. https://doi.org/10.1016/j.semcancer.2021.02.014
Sottoriva A, Spiteri I, Piccirillo SGM et al (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci 110:4009–4014. https://doi.org/10.1073/pnas.1219747110
Becker AP, Sells BE, Haque SJ, Chakravarti A (2021) Tumor heterogeneity in Glioblastomas: from light Microscopy to Molecular Pathology. Cancers 13:761. https://doi.org/10.3390/cancers13040761
Parker NR, Khong P, Parkinson JF et al (2015) Molecular Heterogeneity in Glioblastoma: potential clinical implications. Front Oncol 5
McGillicuddy LT, Fromm JA, Hollstein PE et al (2009) Proteasomal and genetic inactivation of the NF1 tumor suppressor in gliomagenesis. Cancer Cell 16:44–54. https://doi.org/10.1016/j.ccr.2009.05.009
Herting CJ, Chen Z, Pitter KL et al (2017) Genetic driver mutations define the expression signature and microenvironmental composition of high-grade gliomas. Glia 65:1914–1926. https://doi.org/10.1002/glia.23203
Chen J, Wu L, Yang H et al (2022) Establishment of three heterogeneous subtypes and a risk model of low-grade gliomas based on cell senescence-related genes. Front Immunol 13:982033. https://doi.org/10.3389/fimmu.2022.982033
Kim EL, Sorokin M, Kantelhardt SR et al (2020) Intratumoral Heterogeneity and Longitudinal Changes in Gene expression predict Differential Drug Sensitivity in newly diagnosed and recurrent glioblastoma. Cancers 12:520. https://doi.org/10.3390/cancers12020520
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of Tumours of the Central Nervous System. Acta Neuropathol (Berl) 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Collado M, Gil J, Efeyan A et al (2005) Senescence in premalignant tumours. Nature 436:642–642. https://doi.org/10.1038/436642a
Collado M, Serrano M (2010) Senescence in tumours: evidence from mice and humans. Nat Rev Cancer 10:51–57. https://doi.org/10.1038/nrc2772
Brennan CW, Verhaak RGW, McKenna A et al (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. https://doi.org/10.1016/j.cell.2013.09.034
Ishii N, Maier D, Merlo A et al (2006) Frequent co-alterations of TP53, p16/CDKN2A, p14 ARF, PTEN Tumor suppressor genes in human glioma cell lines. Brain Pathol 9:469–479. https://doi.org/10.1111/j.1750-3639.1999.tb00536.x
Ohgaki H, Dessen P, Jourde B et al (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64:6892–6899. https://doi.org/10.1158/0008-5472.CAN-04-1337
Ohgaki H, Kleihues P (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170:1445–1453. https://doi.org/10.2353/ajpath.2007.070011
McClelland S, Hall WA (2007) Postoperative central nervous system infection: incidence and Associated factors in 2111 neurosurgical procedures. Clin Infect Dis 45:55–59. https://doi.org/10.1086/518580
Brooks CL, Gu W (2006) p53 ubiquitination: Mdm2 and beyond. Mol Cell 21:307–315. https://doi.org/10.1016/j.molcel.2006.01.020
Reifenberger G, Liu L, Ichimura K et al (1993) Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res 53:2736–2739
Biernat W, Kleihues P, Yonekawa Y, Ohgaki H (1997) Amplification and overexpression of MDM2 in primary (de novo) glioblastomas. J Neuropathol Exp Neurol 56:180–185. https://doi.org/10.1097/00005072-199702000-00009
Franovic A, Elliott KC, Seguin L et al (2015) Glioblastomas require integrin αvβ3/PAK4 signaling to escape senescence. Cancer Res 75:4466–4473. https://doi.org/10.1158/0008-5472.CAN-15-0988
He G, Siddik ZH, Huang Z et al (2005) Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 24:2929–2943. https://doi.org/10.1038/sj.onc.1208474
Xue W, Zender L, Miething C et al (2007) Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–660. https://doi.org/10.1038/nature05529
Feng Z, Zhang H, Levine AJ, Jin S (2005) The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci 102:8204–8209. https://doi.org/10.1073/pnas.0502857102
Hafner A, Bulyk ML, Jambhekar A, Lahav G (2019) The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol 20:199–210. https://doi.org/10.1038/s41580-019-0110-x
Williams AB, Schumacher B (2016) p53 in the DNA-Damage-repair process. Cold Spring Harb Perspect Med 6:a026070. https://doi.org/10.1101/cshperspect.a026070
Wang C, Jurk D, Maddick M et al (2009) DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8:311–323. https://doi.org/10.1111/j.1474-9726.2009.00481.x
d’Adda di Fagagna F (2008) Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer 8:512–522. https://doi.org/10.1038/nrc2440
Weichhart T (2018) mTOR as regulator of lifespan, aging and cellular senescence. Gerontology 64:127–134. https://doi.org/10.1159/000484629
Bykov VJN, Wiman KG (2014) Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett 588:2622–2627. https://doi.org/10.1016/j.febslet.2014.04.017
Forte IM, Indovina P, Iannuzzi CA et al (2019) Targeted therapy based on p53 reactivation reduces both glioblastoma cell growth and resistance to temozolomide. Int J Oncol 54:2189–2199. https://doi.org/10.3892/ijo.2019.4788
Issaeva N, Bozko P, Enge M et al (2004) Small molecule RITA binds to p53, blocks p53–HDM-2 interaction and activates p53 function in tumors. Nat Med 10:1321–1328. https://doi.org/10.1038/nm1146
Bykov VJN, Issaeva N, Shilov A et al (2002) Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med 8:282–288. https://doi.org/10.1038/nm0302-282
Lambert JMR, Gorzov P, Veprintsev DB et al (2009) PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell 15:376–388. https://doi.org/10.1016/j.ccr.2009.03.003
Zhang Q, Bykov VJN, Wiman KG, Zawacka-Pankau J (2018) APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis 9:439. https://doi.org/10.1038/s41419-018-0463-7
Weinmann L, Wischhusen J, Demma MJ et al (2008) A novel p53 rescue compound induces p53-dependent growth arrest and sensitises glioma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ 15:718–729. https://doi.org/10.1038/sj.cdd.4402301
De La Rosa J, Urdiciain A, Zelaya MV et al (2021) APR-246 combined with 3-deazaneplanocin A, panobinostat or temozolomide reduces clonogenicity and induces apoptosis in glioblastoma cells. Int J Oncol 58:312–330. https://doi.org/10.3892/ijo.2021.5177
Pagliaro LC, Keyhani A, Williams D et al (2003) Repeated intravesical instillations of an adenoviral vector in patients with locally advanced bladder cancer: a phase I study of p53 gene therapy. J Clin Oncol Off J Am Soc Clin Oncol 21:2247–2253. https://doi.org/10.1200/JCO.2003.09.138
Qazilbash MH, Xiao X, Seth P et al (1997) Cancer gene therapy using a novel adeno-associated virus vector expressing human wild-type p53. Gene Ther 4:675–682. https://doi.org/10.1038/sj.gt.3300444
Lang FF, Bruner JM, Fuller GN et al (2003) Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol Off J Am Soc Clin Oncol 21:2508–2518. https://doi.org/10.1200/JCO.2003.21.13.2508
Badie B, Drazan KE, Kramar MH et al (1995) Adenovirus-mediated p53 gene delivery inhibits 9L glioma growth in rats. Neurol Res 17:209–216. https://doi.org/10.1080/01616412.1995.11740314
Kleinstiver BP, Pattanayak V, Prew MS et al (2016) High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529:490–495. https://doi.org/10.1038/nature16526
Shalem O, Sanjana NE, Zhang F (2015) High-throughput functional genomics using CRISPR–Cas9. Nat Rev Genet 16:299–311. https://doi.org/10.1038/nrg3899
Zou Y, Sun X, Yang Q et al (2022) Blood-brain barrier–penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci Adv 8:eabm8011. https://doi.org/10.1126/sciadv.abm8011
Kim S-S, Rait A, Kim E et al (2014) A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano 8:5494–5514. https://doi.org/10.1021/nn5014484
Fan X, Lu H, Cui Y et al (2018) Overexpression of p53 delivered using recombinant NDV induces apoptosis in glioma cells by regulating the apoptotic signaling pathway. Exp Ther Med 15:4522–4530. https://doi.org/10.3892/etm.2018.5935
Inaba N, Kimura M, Fujioka K et al (2011) The effect of PTEN on proliferation and drug-, and radiosensitivity in malignant glioma cells. Anticancer Res 31:1653–1658
Banerjee K, Núñez FJ, Haase S et al (2021) Current approaches for Glioma Gene Therapy and Virotherapy. Front Mol Neurosci 14
Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137:413–431. https://doi.org/10.1016/j.cell.2009.04.037
Lavin MF, Gueven N (2006) The complexity of p53 stabilization and activation. Cell Death Differ 13:941–950. https://doi.org/10.1038/sj.cdd.4401925
Dai C, Gu W (2010) p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med 16:528–536. https://doi.org/10.1016/j.molmed.2010.09.002
Costa B, Bendinelli S, Gabelloni P et al (2013) Human Glioblastoma Multiforme: p53 reactivation by a novel MDM2 inhibitor. PLoS ONE 8:e72281. https://doi.org/10.1371/journal.pone.0072281
Villalonga-Planells R, Coll-Mulet L, Martínez-Soler F et al (2011) Activation of p53 by Nutlin-3a induces apoptosis and Cellular Senescence in Human Glioblastoma Multiforme. PLoS ONE 6:e18588. https://doi.org/10.1371/journal.pone.0018588
Suzuki H, Aoki K, Chiba K et al (2015) Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet 47:458–468. https://doi.org/10.1038/ng.3273
Kovatcheva M, Liao W, Klein ME et al (2017) ATRX is a regulator of therapy induced senescence in human cells. Nat Commun 8:386. https://doi.org/10.1038/s41467-017-00540-5
Krtolica A, Parrinello S, Lockett S et al (2001) Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci 98:12072–12077. https://doi.org/10.1073/pnas.211053698
Hirose Y, Berger MS, Pieper RO (2001) p53 effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res 61:1957–1963
Knizhnik AV, Roos WP, Nikolova T et al (2013) Survival and death strategies in Glioma cells: Autophagy, Senescence and apoptosis triggered by a single type of Temozolomide-Induced DNA damage. PLoS ONE 8:e55665. https://doi.org/10.1371/journal.pone.0055665
Aasland D, Götzinger L, Hauck L et al (2019) Temozolomide induces senescence and repression of DNA repair pathways in Glioblastoma cells via activation of ATR–CHK1, p21, and NF-κB. Cancer Res 79:99–113. https://doi.org/10.1158/0008-5472.CAN-18-1733
Quiros S, Roos WP, Kaina B (2010) Processing of O6-methylguanine into DNA double-strand breaks requires two rounds of replication whereas apoptosis is also induced in subsequent cell cycles. Cell Cycle Georget Tex 9:168–178. https://doi.org/10.4161/cc.9.1.10363
Yoshioka K, Yoshioka Y, Hsieh P (2006) ATR kinase activation mediated by MutSα and MutLα in response to cytotoxic O6-Methylguanine adducts. Mol Cell 22:501–510. https://doi.org/10.1016/j.molcel.2006.04.023
Zhao J, Zhang L, Lu A et al (2020) ATM is a key driver of NF-κB-dependent DNA-damage-induced senescence, stem cell dysfunction and aging. Aging 12:4688–4710. https://doi.org/10.18632/aging.102863
Eich M, Roos WP, Nikolova T, Kaina B (2013) Contribution of ATM and ATR to the resistance of glioblastoma and malignant melanoma cells to the methylating anticancer drug temozolomide. Mol Cancer Ther 12:2529–2540. https://doi.org/10.1158/1535-7163.MCT-13-0136
Schwarzenbach C, Tatsch L, Brandstetter Vilar J et al (2021) Targeting c-IAP1, c-IAP2, and Bcl-2 eliminates senescent glioblastoma cells following Temozolomide Treatment. Cancers 13:3585. https://doi.org/10.3390/cancers13143585
Jinno-Oue A, Shimizu N, Hamada N et al (2010) Irradiation with carbon ion beams induces apoptosis, autophagy, and cellular senescence in a human glioma-derived cell line. Int J Radiat Oncol Biol Phys 76:229–241. https://doi.org/10.1016/j.ijrobp.2009.08.054
Quick QA, Gewirtz DA (2006) An accelerated senescence response to radiation in wild-type p53 glioblastoma multiforme cells. J Neurosurg 105:111–118. https://doi.org/10.3171/jns.2006.105.1.111
Ye L, Wang C, Yu G et al (2014) Bmi-1 induces radioresistance by suppressing senescence in human U87 glioma cells. Oncol Lett 8:2601–2606. https://doi.org/10.3892/ol.2014.2606
Broestl L, Warrington NM, Grandison L et al (2022) Gonadal sex patterns p21-induced cellular senescence in mouse and human glioblastoma. Commun Biol 5:1–18. https://doi.org/10.1038/s42003-022-03743-9
Zanoni M, Sarti AC, Zamagni A et al (2022) Irradiation causes senescence, ATP release, and P2X7 receptor isoform switch in glioblastoma. Cell Death Dis 13:1–14. https://doi.org/10.1038/s41419-022-04526-0
Kaur E, Rajendra J, Jadhav S et al (2015) Radiation-induced homotypic cell fusions of innately resistant glioblastoma cells mediate their sustained survival and recurrence. Carcinogenesis 36:685–695. https://doi.org/10.1093/carcin/bgv050
Chen Z, Cao K, Xia Y et al (2019) Cellular senescence in ionizing radiation (review). Oncol Rep 42:883–894. https://doi.org/10.3892/or.2019.7209
Turnquist C, Beck JA, Horikawa I et al (2019) Radiation-induced astrocyte senescence is rescued by ∆133p53. Neuro-Oncol 21:474–485. https://doi.org/10.1093/neuonc/noz001
Jeon H-Y, Kim J-K, Ham SW et al (2016) Irradiation induces glioblastoma cell senescence and senescence-associated secretory phenotype. Tumour Biol J Int Soc Oncodevelopmental Biol Med 37:5857–5867. https://doi.org/10.1007/s13277-015-4439-2
Limbad C, Oron TR, Alimirah F et al (2020) Astrocyte senescence promotes glutamate toxicity in cortical neurons. PLoS ONE 15:e0227887. https://doi.org/10.1371/journal.pone.0227887
Faget DV, Ren Q, Stewart SA (2019) Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer 19:439–453. https://doi.org/10.1038/s41568-019-0156-2
Sarvaiya PJ, Guo D, Ulasov I et al (2013) Chemokines in tumor progression and metastasis. Oncotarget 4:2171–2185. https://doi.org/10.18632/oncotarget.1426
Fares J, Cordero A, Kanojia D, Lesniak MS (2021) The Network of Cytokines in Brain Metastases. Cancers 13:142. https://doi.org/10.3390/cancers13010142
Coppé J-P, Desprez P-Y, Krtolica A, Campisi J (2010) The Senescence-Associated Secretory phenotype: the Dark side of Tumor suppression. Annu Rev Pathol 5:99–118. https://doi.org/10.1146/annurev-pathol-121808-102144
Gorgoulis V, Adams PD, Alimonti A et al (2019) Cellular Senescence: defining a path Forward. Cell 179:813–827. https://doi.org/10.1016/j.cell.2019.10.005
Ogrodnik M, Evans SA, Fielder E et al (2021) Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 20:e13296. https://doi.org/10.1111/acel.13296
Riessland M, Kolisnyk B, Kim TW et al (2019) Loss of SATB1 induces p21-dependent cellular senescence in post-mitotic dopaminergic neurons. Cell Stem Cell 25:514–530e8. https://doi.org/10.1016/j.stem.2019.08.013
Schwab N, Grenier K, Hazrati L-N (2019) DNA repair deficiency and senescence in concussed professional athletes involved in contact sports. Acta Neuropathol Commun 7:182. https://doi.org/10.1186/s40478-019-0822-3
Yousefzadeh MJ, Zhao J, Bukata C et al (2020) Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19:e13094. https://doi.org/10.1111/acel.13094
Acosta JC, Banito A, Wuestefeld T et al (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15:978–990. https://doi.org/10.1038/ncb2784
Salminen A, Kauppinen A, Kaarniranta K (2012) Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal 24:835–845. https://doi.org/10.1016/j.cellsig.2011.12.006
Orjalo AV, Bhaumik D, Gengler BK et al (2009) Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A 106:17031–17036. https://doi.org/10.1073/pnas.0905299106
Ortiz-Montero P, Londoño-Vallejo A, Vernot J-P (2017) Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal CCS 15:17. https://doi.org/10.1186/s12964-017-0172-3
Acosta JC, O’Loghlen A, Banito A et al (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133:1006–1018. https://doi.org/10.1016/j.cell.2008.03.038
Marin I, Boix O, Garcia-Garijo A et al (2023) Cellular Senescence is immunogenic and promotes Antitumor Immunity. Cancer Discov 13:410–431. https://doi.org/10.1158/2159-8290.CD-22-0523
Iannello A, Thompson TW, Ardolino M et al (2013) p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 210:2057–2069. https://doi.org/10.1084/jem.20130783
Kale A, Sharma A, Stolzing A et al (2020) Role of immune cells in the removal of deleterious senescent cells. Immun Ageing 17:16. https://doi.org/10.1186/s12979-020-00187-9
Ruscetti M, Leibold J, Bott MJ et al (2018) NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 362:1416–1422. https://doi.org/10.1126/science.aas9090
Wang H, Lathia JD, Wu Q et al (2009) Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells Dayt Ohio 27:2393–2404. https://doi.org/10.1002/stem.188
Lamano JB, Lamano JB, Li YD et al (2019) Glioblastoma-derived IL-6 induces immunosuppressive peripheral myeloid cell PD-L1 and promotes Tumor Growth. Clin Cancer Res Off J Am Assoc Cancer Res 25:3643–3657. https://doi.org/10.1158/1078-0432.CCR-18-2402
Sharma I, Singh A, Siraj F, Saxena S (2018) IL-8/CXCR1/2 signalling promotes tumor cell proliferation, invasion and vascular mimicry in glioblastoma. J Biomed Sci 25. https://doi.org/10.1186/s12929-018-0464-y
Kaminska B, Kocyk M, Kijewska M (2013) TGF beta signaling and its role in glioma pathogenesis. Adv Exp Med Biol 986:171–187. https://doi.org/10.1007/978-94-007-4719-7_9
Ikushima H, Todo T, Ino Y et al (2009) Autocrine TGF-β signaling maintains tumorigenicity of glioma-initiating cells through sry-related HMG-Box factors. Cell Stem Cell 5:504–514. https://doi.org/10.1016/j.stem.2009.08.018
Davalos AR, Coppe J-P, Campisi J, Desprez P-Y (2010) Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 29:273–283. https://doi.org/10.1007/s10555-010-9220-9
Fares J, Fares MY, Khachfe HH et al (2020) Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 5:28. https://doi.org/10.1038/s41392-020-0134-x
Salam R, Saliou A, Bielle F et al (2023) Cellular senescence in malignant cells promotes tumor progression in mouse and patient glioblastoma. Nat Commun 14:441. https://doi.org/10.1038/s41467-023-36124-9
Wang B, Kohli J, Demaria M (2020) Senescent cells in Cancer Therapy: friends or foes? Trends Cancer 6:838–857. https://doi.org/10.1016/j.trecan.2020.05.004
Demaria M, O’Leary MN, Chang J et al (2017) Cellular Senescence promotes adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov 7:165–176. https://doi.org/10.1158/2159-8290.CD-16-0241
Watanabe S, Kawamoto S, Ohtani N, Hara E (2017) Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence‐associated diseases. Cancer Sci 108:563–569. https://doi.org/10.1111/cas.13184
Roberson RS, Kussick SJ, Vallieres E et al (2005) Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res 65:2795–2803. https://doi.org/10.1158/0008-5472.CAN-04-1270
Rajaraman R, Guernsey DL, Rajaraman MM, Rajaraman SR (2006) Stem cells, senescence, neosis and self-renewal in cancer. Cancer Cell Int 6:25. https://doi.org/10.1186/1475-2867-6-25
Saleh T, Tyutyunyk-Massey L, Murray GF et al (2019) Tumor cell escape from therapy-induced senescence. Biochem Pharmacol 162:202–212. https://doi.org/10.1016/j.bcp.2018.12.013
Saleh T, Bloukh S, Carpenter VJ et al (2020) Therapy-Induced Senescence: an “Old” friend becomes the enemy. Cancers 12:822. https://doi.org/10.3390/cancers12040822
Saleh T, Tyutyunyk-Massey L, Gewirtz DA (2019) Tumor cell escape from Therapy-Induced Senescence as a model of Disease recurrence after Dormancy. Cancer Res 79:1044–1046. https://doi.org/10.1158/0008-5472.CAN-18-3437
Wang Q, Wu PC, Roberson RS et al (2011) Survivin and escaping in therapy-induced cellular senescence. Int J Cancer 128:1546–1558. https://doi.org/10.1002/ijc.25482
Liu J, Xiao Q, Xiao J et al (2022) Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 7:1–23. https://doi.org/10.1038/s41392-021-00762-6
Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4:68–75
Lehmann J, Narcisi R, Franceschini N et al (2022) WNT/beta-catenin signalling interrupts a senescence-induction cascade in human mesenchymal stem cells that restricts their expansion. Cell Mol Life Sci 79:82. https://doi.org/10.1007/s00018-021-04035-x
Lee Y, Lee J-K, Ahn SH et al (2016) WNT signaling in glioblastoma and therapeutic opportunities. Lab Invest 96:137–150. https://doi.org/10.1038/labinvest.2015.140
Milanovic M, Fan DNY, Belenki D et al (2018) Senescence-associated reprogramming promotes cancer stemness. Nature 553:96–100. https://doi.org/10.1038/nature25167
Le Duff M, Gouju J, Jonchère B et al (2018) Regulation of senescence escape by the cdk4–EZH2–AP2M1 pathway in response to chemotherapy. Cell Death Dis 9:199. https://doi.org/10.1038/s41419-017-0209-y
Song Z, Pan Y, Ling G et al (2017) Escape of U251 glioma cells from temozolomide-induced senescence was modulated by CDK1/survivin signaling. Am J Transl Res 9:2163–2180
Pacifico F, Badolati N, Mellone S et al (2021) Glutamine promotes escape from therapy-induced senescence in tumor cells. Aging 13:20962–20991. https://doi.org/10.18632/aging.203495
Lee S, Schmitt CA (2019) The dynamic nature of senescence in cancer. Nat Cell Biol 21:94–101. https://doi.org/10.1038/s41556-018-0249-2
Saleh T, Gewirtz DA (2022) Considering therapy-induced senescence as a mechanism of tumour dormancy contributing to disease recurrence. Br J Cancer 126:1363–1365. https://doi.org/10.1038/s41416-022-01787-6
Borovski T, Beke P, van Tellingen O et al (2013) Therapy-resistant tumor microvascular endothelial cells contribute to treatment failure in glioblastoma multiforme. Oncogene 32:1539–1548. https://doi.org/10.1038/onc.2012.172
Rahman M, Olson I, Mansour M et al (2022) Selective vulnerability of senescent glioblastoma cells to BCL-XL Inhibition. Mol Cancer Res 20:938–948. https://doi.org/10.1158/1541-7786.MCR-21-0029
Ahmed AU, Auffinger B, Lesniak MS (2013) Understanding glioma stem cells: rationale, clinical relevance and therapeutic strategies. Expert Rev Neurother 13:545–555. https://doi.org/10.1586/ern.13.42
Wang L, Leite de Oliveira R, Wang C et al (2017) High-throughput functional genetic and compound screens identify targets for Senescence induction in Cancer. Cell Rep 21:773–783. https://doi.org/10.1016/j.celrep.2017.09.085
Michaud K, Solomon DA, Oermann E et al (2010) Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res 70:3228–3238. https://doi.org/10.1158/0008-5472.CAN-09-4559
Riess C, Koczan D, Schneider B et al (2021) Cyclin-dependent kinase inhibitors exert distinct effects on patient-derived 2D and 3D glioblastoma cell culture models. Cell Death Discov 7:1–15. https://doi.org/10.1038/s41420-021-00423-1
Zhu Z, Du S, Ding F et al (2016) Ursolic acid attenuates temozolomide resistance in glioblastoma cells by downregulating O(6)-methylguanine-DNA methyltransferase (MGMT) expression. Am J Transl Res 8:3299–3308
Berte N, Lokan S, Eich M et al (2016) Artesunate enhances the therapeutic response of glioma cells to temozolomide by inhibition of homologous recombination and senescence. Oncotarget 7:67235–67250. https://doi.org/10.18632/oncotarget.11972
Wei S, Liu L, Chen Z et al (2020) Artesunate inhibits the mevalonate pathway and promotes glioma cell senescence. J Cell Mol Med 24:276–284. https://doi.org/10.1111/jcmm.14717
Kaverina NV, Kadagidze ZG, Borovjagin AV et al (2018) Tamoxifen overrides autophagy inhibition in beclin-1-deficient glioma cells and their resistance to adenovirus-mediated oncolysis via upregulation of PUMA and BAX. Oncogene 37:6069–6082. https://doi.org/10.1038/s41388-018-0395-9
Vengoji R, Macha MA, Nimmakayala RK et al (2019) Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J Exp Clin Cancer Res CR 38:266. https://doi.org/10.1186/s13046-019-1264-2
Harmouch E, Seitlinger J, Chaddad H et al (2020) Flavagline synthetic derivative induces senescence in glioblastoma cancer cells without being toxic to healthy astrocytes. Sci Rep 10:13750. https://doi.org/10.1038/s41598-020-70820-6
Atif F, Yousuf S, Espinosa-Garcia C et al (2019) Progesterone treatment attenuates glycolytic metabolism and induces senescence in Glioblastoma. Sci Rep 9:988. https://doi.org/10.1038/s41598-018-37399-5
Alza L, Nàger M, Visa A et al (2020) FAK Inhibition induces Glioblastoma Cell Senescence-Like State through p62 and p27. Cancers 12:1086. https://doi.org/10.3390/cancers12051086
Xu X, Shen X, Feng W et al (2020) D-galactose induces senescence of glioblastoma cells through YAP-CDK6 pathway. Aging 12:18501–18521. https://doi.org/10.18632/aging.103819
Zhou W, Wang J, Qi Q et al (2018) Matrine induces senescence of human glioblastoma cells through suppression of the IGF1/PI3K/AKT/p27 signaling pathway. Cancer Med 7:4729–4743. https://doi.org/10.1002/cam4.1720
Lehman NL, O’Donnell JP, Whiteley LJ et al (2012) Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle 11:489–502. https://doi.org/10.4161/cc.11.3.18996
Mannino M, Gomez-Roman N, Hochegger H, Chalmers AJ (2014) Differential sensitivity of glioma stem cells to Aurora kinase A inhibitors: implications for stem cell mitosis and centrosome dynamics. Stem Cell Res 13:135–143. https://doi.org/10.1016/j.scr.2014.05.001
Banasavadi-Siddegowda YK, Welker AM, An M et al (2018) PRMT5 as a druggable target for glioblastoma therapy. Neuro-Oncol 20:753–763. https://doi.org/10.1093/neuonc/nox206
Wu J, Su H, Yu Z et al (2020) Skp2 modulates proliferation, senescence and tumorigenesis of glioma. Cancer Cell Int 20:71. https://doi.org/10.1186/s12935-020-1144-z
Pérès EA, Gérault AN, Valable S et al (2015) Silencing erythropoietin receptor on glioma cells reinforces efficacy of temozolomide and X-rays through senescence and mitotic catastrophe. Oncotarget 6:2101–2119. https://doi.org/10.18632/oncotarget.2937
Filippi-Chiela EC, Vargas JE, Bueno e Silva MM et al (2022) Vincristine promotes differential levels of apoptosis, mitotic catastrophe, and senescence depending on the genetic background of glioblastoma cells. Toxicol In Vitro 85:105472. https://doi.org/10.1016/j.tiv.2022.105472
Zamin LL, Filippi-Chiela EC, Dillenburg-Pilla P et al (2009) Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci 100:1655–1662. https://doi.org/10.1111/j.1349-7006.2009.01215.x
Filippi-Chiela EC, Thomé MP, Bueno e Silva MM et al (2013) Resveratrol abrogates the Temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the Temozolomide-induced senescence in glioma cells. BMC Cancer 13:147. https://doi.org/10.1186/1471-2407-13-147
Castino R, Pucer A, Veneroni R et al (2011) Resveratrol reduces the Invasive Growth and promotes the Acquisition of a long-lasting differentiated phenotype in human glioblastoma cells. J Agric Food Chem 59:4264–4272. https://doi.org/10.1021/jf104917q
Liu Q, Xu X, Zhao M et al (2015) Berberine induces senescence of human glioblastoma cells by downregulating the EGFR-MEK-ERK signaling pathway. Mol Cancer Ther 14:355–363. https://doi.org/10.1158/1535-7163.MCT-14-0634
Maletzki C, Rosche Y, Riess C et al (2017) Deciphering molecular mechanisms of arginine deiminase-based therapy—comparative response analysis in paired human primary and recurrent glioblastomas. Chem Biol Interact 278:179–188. https://doi.org/10.1016/j.cbi.2017.10.007
Fuentes-Fayos AC, G-García ME, Pérez-Gómez JM et al (2023) Metformin and simvastatin exert additive antitumour effects in glioblastoma via senescence-state: clinical and translational evidence. https://doi.org/10.1016/j.ebiom.2023.104484. eBioMedicine 90:
Selt F, Sigaud R, Valinciute G et al (2023) BH3 mimetics targeting BCL-XL impact the senescent compartment of pilocytic astrocytoma. Neuro-Oncol 25:735–747. https://doi.org/10.1093/neuonc/noac199
Merino D, Kelly GL, Lessene G et al (2018) BH3-Mimetic drugs: blazing the trail for New Cancer Medicines. Cancer Cell 34:879–891. https://doi.org/10.1016/j.ccell.2018.11.004
Beltzig L, Christmann M, Kaina B (2022) Abrogation of Cellular Senescence Induced by Temozolomide in Glioblastoma cells: search for Senolytics. Cells 11:2588. https://doi.org/10.3390/cells11162588
Chaib S, Tchkonia T, Kirkland JL (2022) Cellular senescence and senolytics: the path to the clinic. Nat Med 28:1556–1568. https://doi.org/10.1038/s41591-022-01923-y
Reich TR, Schwarzenbach C, Vilar JB et al (2021) Localization matters: nuclear-trapped Survivin sensitizes glioblastoma cells to temozolomide by elevating cellular senescence and impairing homologous recombination. Cell Mol Life Sci CMLS 78:5587–5604. https://doi.org/10.1007/s00018-021-03864-0
Katoh M, Wilmotte R, Belkouch M-C et al (2003) Survivin in brain tumors: an attractive target for immunotherapy. J Neurooncol 64:71–76. https://doi.org/10.1007/BF02700022
Zhang S, Zhang C, Song Y et al (2018) Prognostic role of survivin in patients with glioma. Med (Baltim) 97:e0571. https://doi.org/10.1097/MD.0000000000010571
Tong X, Yang P, Wang K et al (2019) Survivin is a prognostic indicator in glioblastoma and may be a target of microRNA-218. Oncol Lett 18:359–367. https://doi.org/10.3892/ol.2019.10335
Bausart M, Préat V, Malfanti A (2022) Immunotherapy for glioblastoma: the promise of combination strategies. J Exp Clin Cancer Res 41:35. https://doi.org/10.1186/s13046-022-02251-2
Yu MW, Quail DF (2021) Immunotherapy for Glioblastoma: current Progress and Challenges. Front Immunol 12
Crane CA, Austgen K, Haberthur K et al (2014) Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc Natl Acad Sci U S A 111:12823–12828. https://doi.org/10.1073/pnas.1413933111
Chibaya L, Snyder J, Ruscetti M (2022) Senescence and the tumor-immune landscape: implications for cancer immunotherapy. Semin Cancer Biol 86:827–845. https://doi.org/10.1016/j.semcancer.2022.02.005
Rossi M, Abdelmohsen K (2021) The emergence of senescent surface biomarkers as senotherapeutic targets. Cells 10:1740. https://doi.org/10.3390/cells10071740
Sagiv A, Burton DGA, Moshayev Z et al (2016) NKG2D ligands mediate immunosurveillance of senescent cells. Aging 8:328–344. https://doi.org/10.18632/aging.100897
Wang T-W, Johmura Y, Suzuki N et al (2022) Blocking PD-L1–PD-1 improves senescence surveillance and ageing phenotypes. Nature 611:358–364. https://doi.org/10.1038/s41586-022-05388-4
Amor C, Feucht J, Leibold J et al (2020) Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583:127–132. https://doi.org/10.1038/s41586-020-2403-9
Huang B, Zhang J, Zong W et al (2023) Myeloidcells in the immunosuppressive microenvironment in glioblastoma: the characteristics and therapeutic strategies. Front Immunol 14
Li K, Shi H, Zhang B et al (2021) Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther 6:1–25. https://doi.org/10.1038/s41392-021-00670-9
Kamran N, Kadiyala P, Saxena M et al (2017) Immunosuppressive myeloid cells’ blockade in the Glioma Microenvironment enhances the efficacy of Immune-Stimulatory Gene Therapy. Mol Ther 25:232–248. https://doi.org/10.1016/j.ymthe.2016.10.003
Zhang L, Pitcher LE, Yousefzadeh MJ et al (2022) Cellular senescence: a key therapeutic target in aging and diseases. J Clin Invest 132:e158450. https://doi.org/10.1172/JCI158450
Short S, Fielder E, Miwa S, von Zglinicki T (2019) Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine 41:683–692. https://doi.org/10.1016/j.ebiom.2019.01.056
Acknowledgements
Final paper assembly and editing as well as approval of the final submitted version of manuscript was done by all authors. Figures are original and were created with Biorender.com.
Funding
National Cancer Institute (NCI): R01NS115955 and P50CA221747.
Author information
Authors and Affiliations
Contributions
All authors contributed to conceptualization, visualization, writing—original draft, and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chojak, R., Fares, J., Petrosyan, E. et al. Cellular senescence in glioma. J Neurooncol 164, 11–29 (2023). https://doi.org/10.1007/s11060-023-04387-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04387-3