Abstract
Importance
High-grade gliomas (HGG) are the most aggressive and common malignant brain tumors in adults. They have a dismally fatal prognosis. Even if gross total resection of the enhancing tumor is achieved, inevitably, invading tumor cells that are indistinguishable to the un-aided eye are left behind, which eventually leads to tumor recurrence. 5-aminolevulinic acid (5-ALA) is an increasingly utilized intraoperative fluorescent imaging agent for patients with HGG. It enhances visualization of HGG tissue. Despite early promising randomized clinical trial data suggesting a survival benefit for 5-ALA-guided surgery, the growing body of literature must be analyzed to confirm efficacy on patient outcomes.
Objective
To perform a systematic review of the literature to evaluate whether there is a beneficial effect upon survival and extent of resection due to the utilization of 5-ALA in HGG surgery.
Evidence review
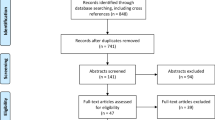
Literature regarding 5-ALA usage in HGG surgery was reviewed according to the PRISMA guidelines. Two databases, PubMed and SCOPUS, were searched for assorted combinations of the keywords “5-ALA,” “high-grade glioma,” “5-aminolevulinic acid,” and “resection” in July 2020 for case reports and retrospective, prospective, and randomized clinical trials assessing and analyzing 5-ALA intraoperative use in patients with HGG. Entailed studies on PubMed and SCOPUS were found for screening using a snowball search technique upon the initially searched papers. Systematic reviews and meta-analyses were excluded from our PRISMA table.
Findings
3756 previously published studies were screened, 536 of which were further evaluated, and ultimately 45 were included in our systematic review. There were no date restrictions on the screened publications. Our literature search was finalized on July 16, 2020. We found an observed increase in the overall survival (OS) and progression-free survival (PFS) of the 5-ALA group compared to the white light group, as well as an observed increase in the OS and PFS of complete resections compared to incomplete resections. Of the studies that directly compared the use of 5-ALA to white light (13 of the total analyzed 45, or 28.9%), 5-ALA lead to a better PFS and OS in 88.4 and 67.5% of patients, respectively.
When the studies that reported postoperative neurologic outcomes of surgeries using 5-ALA vs. white light were analyzed, 42.2% of subjects demonstrated 5-ALA use was associated with less post-op neurological deficits, whereas 34.5% demonstrated no difference between 5-ALA and without. 23.3% of studies showed that intraoperative 5-ALA guided surgeries lead to more post-op neurological deficits.
Conclusions and relevance
Utilization of 5-ALA was found to be associated with a greater extent of resection in HGG surgeries, as well as longer OS and PFS. Postop neurologic deficit rates were mixed and inconclusive when comparing 5-ALA groups to white light groups. 5-ALA is a useful surgical adjunct for resection of HGG when patient safety is preserved.

Similar content being viewed by others
Data availability
The data that support the findings of this study are openly available at https://pubmed.ncbi.nlm.nih.gov and https://www.scopus.com/home.uri.
Abbreviations
- HGG:
-
High grade glioma
- 5-ALA:
-
δ-Aminolevulinic acid
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- FDA:
-
Food and drug administration
- GBM:
-
Glioblastoma multiforme
- EMA:
-
European medicine’s agency
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- WHO:
-
World Health Organization
- MD:
-
Mean difference
- CI:
-
Confidence interval
- iMRI:
-
Intraoperative magnetic resonance imaging
- CEUS:
-
Contrast-enhanced ultrasound
- ioUS:
-
Intraoperative ultrasound
- iCT:
-
Intraoperative computed tomography scan
- EOR:
-
Extent of resection
- GTR:
-
Gross total resection
References
Hadjipanayis CG, Stummer W (2019) 5-ALA and FDA approval for glioma surgery. J Neurooncol 141(3):479–486
Stummer W et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5):392–401
Lacroix M et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95(2):190–198
Beller EM et al (2013) PRISMA for Abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med 10(4):e1001419
Stepp H et al (2007) ALA and malignant glioma: fluorescence-guided resection and photodynamic treatment. J Environ Pathol Toxicol Oncol 26(2):157–164
Kim SK et al (2014) Impact of fluorescence-guided surgery on the improvement of clinical outcomes in glioblastoma patients. Neuro-Oncol Pract 1(3):81–85
Picart T et al (2017) Is fluorescence-guided surgery with 5-ala in eloquent areas for malignant gliomas a reasonable and useful technique? Neurochirurgie 63(3):189–196
Slotty PJ et al (2013) The impact of improved treatment strategies on overall survival in glioblastoma patients. Acta Neurochir 155(6):959–963
Stummer W et al (2011) Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study: clinical article. J Neurosurg 114(3):613–623
Díez Valle R et al (2014) Observational, retrospective study of the effectiveness of 5-aminolevulinic acid in malignant glioma surgery in Spain (The VISIONA study). Neurologia 29(3):131–138
Ng WP et al (2017) Fluorescence-guided versus conventional surgical resection of high grade glioma: a single-centre, 7-year, comparative effectiveness study. Malays J Med Sci 24(2):78–86
Eljamel MS, Goodman C, Moseley H (2008) ALA and photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre phase III randomised controlled trial. Lasers Med Sci 23(4):361–367
Eyüpoglu IY et al (2016) Supra-complete surgery via dual intraoperative visualization approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget 7(18):25755
Hauser SB et al (2016) Combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery: a histology-based evaluation. Neurosurgery 78(4):475–483
Hickmann A-K, Nadji-Ohl M, Hopf NJ (2015) Feasibility of fluorescence-guided resection of recurrent gliomas using five-aminolevulinic acid: retrospective analysis of surgical and neurological outcome in 58 patients. J Neurooncol 122(1):151–160
Jacquesson T et al (2013) Surgery of high-grade gliomas guided by fluorescence: a retrospective study of 22 patients. Neurochirurgie 59(1):9–16
Aldave G et al (2013) Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic acid-guided surgery. Neurosurgery 72(6):915–920
Pichlmeier U et al (2008) Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol 10(6):1025–1034
Stummer W et al (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93(6):1003–1013
Panciani PP et al (2012) Fluorescence and image guided resection in high grade glioma. Clin Neurol Neurosurg 114(1):37–41
Díez Valle R, Tejada Solis S (2015) To what extent will 5-aminolevulinic acid change the face of malignant glioma surgery? CNS Oncol 4(4):265–272
Della Puppa A et al (2014) 5-Aminolevulinic acid fluorescence in high grade glioma surgery: surgical outcome, intraoperative findings, and fluorescence patterns. Biomed Res Int 2014:232561
Della Puppa A et al (2013) 5-aminolevulinic acid (5-ALA) fluorescence guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. Our experience and review of the literature. Acta Neurochir 155(6):965–972
Díez Valle R et al (2011) Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neurooncol 102(1):105–113
Idoate MA et al (2011) Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology 31(6):575–582
Hefti M et al (2008) 5-Aminolaevulinic acid-induced protoporphyrin IX fluorescence in high-grade glioma surgery. Swiss Med Wkly 138(11–12):180–185
Schucht P et al (2012) Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery 71(5):927–935
Teixidor P et al (2016) Safety and efficacy of 5-aminolevulinic acid for high grade glioma in usual clinical practice: a prospective cohort study. PLoS ONE 11(2):e0149244
Eriksson M et al (2019) Improved treatment of glioblastoma—changes in survival over two decades at a single regional centre. Acta Oncol 58(3):334–341
Nabavi A et al (2009) Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery 65(6):1070–1076
Chan DTM, Yi-Pin Sonia H, Poon WS (2018) 5-Aminolevulinic acid fluorescence guided resection of malignant glioma: Hong Kong experience. Asian J Surg 41(5):467–472
Cordova JS et al (2016) Semi-automated volumetric and morphological assessment of glioblastoma resection with fluorescence-guided surgery. Mol Imaging Biol 18(3):454–462
Cortnum S, Laursen RJ (2012) Fluorescence-guided resection of gliomas. Dan Med J 59(8):A4460
Pastor J et al (2013) Role of intraoperative neurophysiological monitoring during fluorescence-guided resection surgery. Acta Neurochir 155(12):2201–2213
Feigl GC et al (2010) Resection of malignant brain tumors in eloquent cortical areas: A new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. J Neurosurg 113(2):352–357
Piquer J et al (2014) Fluorescence-guided surgery and biopsy in gliomas with an exoscope system. BioMed Res Int 2014:207974
Stummer W et al (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62(3):564–576
Tykocki T et al (2012) Fluorescence-guided resection of primary and recurrent malignant gliomas with 5-aminolevulinic acid. Preliminary results. Neurol Neurochir Pol 46(1):47–51
Barbagallo GMV et al (2016) Portable intraoperative computed tomography scan in image-guided surgery for brain high-grade gliomas: analysis of technical feasibility and impact on extent of tumor resection. Oper Neurosurg 12(1):19–30
Coburger J et al (2015) Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS ONE 10(6):e0131872
Eyüpoglu IY et al (2012) Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS ONE 7(9):e44885
Nickel K et al (2018) The patients’ view: impact of the extent of resection, intraoperative imaging, and awake surgery on health-related quality of life in high-grade glioma patients—results of a multicenter cross-sectional study. Neurosurg Rev 41(1):207–219
Tsugu A et al (2011) Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurgery 76(1):120–127
Roder C et al (2014) Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 40(3):297–304
Schatlo B et al (2015) Outcomes after combined use of intraoperative MRI and 5-aminolevulinic acid in high-grade glioma surgery. Neuro Oncol 17(12):1560–1567
Yamada S et al (2015) Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clin Neurol Neurosurg 130:134–139
Della Pepa GM et al (2020) 5-Aminolevulinic acid and contrast-enhanced ultrasound: the combination of the two techniques to optimize the extent of resection in glioblastoma surgery. Neurosurgery 86(6):E529–E540
Neidert MC et al (2016) The influence of intraoperative resection control modalities on survival following gross total resection of glioblastoma. Neurosurg Rev 39(3):401–409
Colapaoli L et al (2006) A case of anaphylactic shock possibly caused by intravesical hexvix. Acta Anaesthesiol Scand 50(9):1165–1167
Kreth FW et al (2013) Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol 24(12):3117–3123
Nader S et al (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115(1):3–8
Schucht P et al (2014) 5-ALA complete resections go beyond MR contrast enhancement: shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir 156(2):305–312
Funding
This systematic review received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material, preparation, data collection, and analysis were performed by TE, DE, and VML. The first draft of the manuscript was written by TE; DGE and MEI commented/edited and VML executed statistical/data analysis. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The following authors have no financial disclosures or personal conflicts of interest: TAE, DE, VL, LD, RJK, MEI.
Ethical approval
Ethical approval was not applicable for this study as only publicly accessible data was utilized.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eatz, T.A., Eichberg, D.G., Lu, V.M. et al. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: a systematic review. J Neurooncol 156, 233–256 (2022). https://doi.org/10.1007/s11060-021-03901-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03901-9