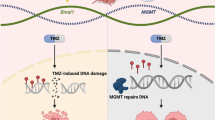
Abstract
Background
Radiotherapy-induced tumor death remains critical in the successful first-line management of glioblastoma, whereas resistance to radiation serves as a major factor in disease progression. Mesenchymal shift has been identified as a driver in GBM recurrence, with gene expression associated with enhanced repair of macromolecular damage caused by radiation.
Methods
Using distinct mesenchymal subtype GBM cells lines, radiation response was assessed by clonogenic assay and orthotopic mouse tumor model. RNA-sequencing was performed in the setting of increasing radiation dosing while real-time assessment of ROS generation was achieved by the measurement of hydroxyl spin trap adducts via electron paramagnetic resonance.
Results
Radiation-induced cell death determined by clonogenic assay was significantly different at low dose (4-8 Gy) between the resistant U3035 cells and the sensitive U3020 cells. Similar trends were present in the in vivo NSG mouse model following radiation dosing on post-implantation day 7–10, with the rate of reduction in tumor bioluminescence reversing between the U3020 and U3035 cells after the third dose of radiation. Changes in gene expression following radiation determined by RNA-sequencing indicate both U3035 and U3020 cells demonstrate a shift toward more mesenchymal profiles, with concurrent shift away from pro-neural subtype gene expression in the U3020 cells that appeared to develop resistance to radiation in vivo. Persistence of ROS generated following radiation was greater in U3020 cells shown to be more sensitive to radiation.
Conclusions
Despite the same molecular classification, distinct GBM cell lines can demonstrate differential response to radiation and potential for mesenchymal shift associated with radiation resistance.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Holou WN, Hodges TR, Everson RG, Freeman J, Zhou S, Suki D, Rao G, Ferguson SD, Heimberger AB, McCutcheon IE, Prabhu SS, Lang FF, Weinberg JS, Wildrick DM, Sawaya R (2020) Perilesional resection of glioblastoma is independently associated with improved outcomes. Neurosurgery 86:112–121. https://doi.org/10.1093/neuros/nyz008
Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318:2306–2316. https://doi.org/10.1001/jama.2017.18718
Certo F, Stummer W, Farah JO, Freyschlag C, Visocchi M, Morrone A, Altieri R, Toccaceli G, Peschillo S, Thomè C, Jenkinson M, Barbagallo G (2019) Supramarginal resection of glioblastoma: 5-ALA fluorescence, combined intraoperative strategies and correlation with survival. J NeurosurgSci 63:625–632
Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA, Sanders CM, Kawaguchi ES, Du L, Li G, Yong WH, Gaffey SC, Cohen AL, Mellinghoff IK, Lee EQ, Reardon DA, O’Brien BJ, Butowski NA, Nghiemphu PL, Clarke JL, Arrillaga-Romany IC, Colman H, Kaley TJ, de Groot JF, Liau LM, Wen PY, Prins RM (2019) Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 25:477–486. https://doi.org/10.1038/s41591-018-0337-7
Sun MZ, Oh T, Ivan ME, Clark AJ, Safaee M, Sayegh ET, Kaur G, Parsa AT, Bloch O (2015) Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg 122:1144–1150. https://doi.org/10.3171/2014.9.JNS14193
Gurbani S, Weinberg B, Cooper L, Mellon E, Schreibmann E, Sheriff S, Maudsley A, Goryawala M, Shu HK, Shim H (2019) The brain imaging collaboration suite (Br ICS): a cloud platform for integrating whole-brain spectroscopic MRI into the radiation therapy planning workflow. Tomography 5:184–191
Niyazi M, Brada M, Chalmers AJ, Combs SE, Erridge SC, Fiorentino A, Grosu AL, Lagerwaard FJ, Minniti G, Mirimanoff RO, Ricardi U, Short SC, Weber DC, Belka C (2016) ESTRO-ACROP guideline “target delineation of glioblastomas.” Radiother Oncol 118:35–42. https://doi.org/10.1016/j.radonc.2015.12.003
Sarria GR, Sperk E, Han X, Sarria GJ, Wenz F, Brehmer S, Fu B, Min S, Zhang H, Qin S, Qiu X, Hänggi D, Abo-Madyan Y, Martinez D, Cabrera C, Giordano FA (2020) Intraoperative radiotherapy for glioblastoma: an international pooled analysis. Radiother Oncol 142:162–167. https://doi.org/10.1016/j.radonc.2019.09.023
Gessler DJ, Ferreira C, Dusenbery K, Chen CC (2020) GammaTile. Future Oncol. https://doi.org/10.2217/fon-2020-0558
Wernicke AG, Yondorf MZ, Peng L, Trichter S, Nedialkova L, Sabbas A, Kulidzhanov F, Parashar B, Nori D, Clifford Chao KS, Christos P, Kovanlikaya I, Pannullo S, Boockvar JA, Stieg PE, Schwartz TH (2014) Phase I/II study of resection and intraoperative cesium-131 radioisotope brachytherapy in patients with newly diagnosed brain metastases. J Neurosurg 121:338–348. https://doi.org/10.3171/2014.3.JNS131140
Carrie C, Kieffer V, Figarella-Branger D, Masliah-Planchon J, Bolle S, Bernier V, Laprie A, Supiot S, Leseur J, Habrand JL, Alapetite C, Kerr C, Dufour C, Claude L, Chapet S, Huchet A, Bondiau PY, Escande A, Truc G, Nguyen TD, Pasteuris C, Vigneron C, Muracciole X, Bourdeaut F, Appay R, Dubray B, Colin C, Ferlay C, Dussart S, Chabaud S, Padovani L, (GFRP) FGoPR, (SFCE) FSoPC (2020) Exclusive hyperfractionated radiation therapy and reduced boost volume for standard-risk medulloblastoma: pooled analysis of the 2 French multicentric studies MSFOP98 and MSFOP 2007 and correlation with molecular subgroups. Int J RadiatOncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2020.07.2324
Kline CN, Joseph NM, Grenert JP, van Ziffle J, Talevich E, Onodera C, Aboian M, Cha S, Raleigh DR, Braunstein S, Torkildson J, Samuel D, Bloomer M, Campomanes AGA, Banerjee A, Butowski N, Raffel C, Tihan T, Bollen AW, Phillips JJ, Korn WM, Yeh I, Bastian BC, Gupta N, Mueller S, Perry A, Nicolaides T, Solomon DA (2017) Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro Oncol 19:699–709. https://doi.org/10.1093/neuonc/now254
Djuzenova CS, Elsner I, Katzer A, Worschech E, Distel LV, Flentje M, Polat B (2013) Radiosensitivity in breast cancer assessed by the histone γ-H2AX and 53BP1 foci. Radiat Oncol 8:98. https://doi.org/10.1186/1748-717X-8-98
Mariotti LG, Pirovano G, Savage KI, Ghita M, Ottolenghi A, Prise KM, Schettino G (2013) Use of the γ-H2AX assay to investigate DNA repair dynamics following multiple radiation exposures. PLoS ONE 8:e79541. https://doi.org/10.1371/journal.pone.0079541
Lê S, Josse J (2008) Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:18
Morokoff A, Ng W, Gogos A, Kaye AH (2015) Molecular subtypes, stem cells and heterogeneity: implications for personalised therapy in glioma. J Clin Neurosci 22:1219–1226. https://doi.org/10.1016/j.jocn.2015.02.008
Bergman D, Modh A, Schultz L, Snyder J, Mikkelsen T, Shah M, Ryu S, Siddiqui MS, Walbert T (2020) Randomized prospective trial of fractionated stereotactic radiosurgery with chemotherapy versus chemotherapy alone for bevacizumab-resistant high-grade glioma. J Neurooncol 148:353–361. https://doi.org/10.1007/s11060-020-03526-4
Chapman CH, Hara JH, Molinaro AM, Clarke JL, Oberheim Bush NA, Taylor JW, Butowski NA, Chang SM, Fogh SE, Sneed PK, Nakamura JL, Raleigh DR, Braunstein SE (2019) Reirradiation of recurrent high-grade glioma and development of prognostic scores for progression and survival. Neurooncol Pract 6:364–374. https://doi.org/10.1093/nop/npz017
Wernicke AG, Taube S, Smith AW, Herskovic A, Parashar B, Schwartz TH (2020) Cs-131 brachytherapy for patients with recurrent glioblastoma combined with bevacizumab avoids radiation necrosis while maintaining local control. Brachytherapy 19:705–712. https://doi.org/10.1016/j.brachy.2020.06.013
Behnan J, Finocchiaro G, Hanna G (2019) The landscape of the mesenchymal signature in brain tumours. Brain 142:847–866. https://doi.org/10.1093/brain/awz044
Guardiola C, Prezado Y, Roulin C, Bergs JWJ (2018) Effect of X-ray minibeam radiation therapy on clonogenic survival of glioma cells. Clin Transl Radiat Oncol 13:7–13. https://doi.org/10.1016/j.ctro.2018.07.005
Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavaré S (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A 110:4009–4014. https://doi.org/10.1073/pnas.1219747110
Segerman A, Niklasson M, Haglund C, Bergström T, Jarvius M, Xie Y, Westermark A, Sönmez D, Hermansson A, Kastemar M, Naimaie-Ali Z, Nyberg F, Berglund M, Sundström M, Hesselager G, Uhrbom L, Gustafsson M, Larsson R, Fryknäs M, Segerman B, Westermark B (2016) Clonal variation in drug and radiation response among glioma-initiating cells is linked to proneural-mesenchymal transition. Cell Rep 17:2994–3009. https://doi.org/10.1016/j.celrep.2016.11.056
Fedele M, Cerchia L, Pegoraro S, Sgarra R, Manfioletti G (2019) Proneural-mesenchymal transition: phenotypic plasticity to acquire multitherapy resistance in glioblastoma. Int J MolSci. https://doi.org/10.3390/ijms20112746
Halliday J, Helmy K, Pattwell SS, Pitter KL, LaPlant Q, Ozawa T, Holland EC (2014) In vivo radiation response of proneural glioma characterized by protective p53 transcriptional program and proneural-mesenchymal shift. Proc Natl Acad Sci U S A 111:5248–5253. https://doi.org/10.1073/pnas.1321014111
Rana S, Chawla R, Kumar R, Singh S, Zheleva A, Dimitrova Y, Gadjeva V, Arora R, Sultana S, Sharma RK (2010) Electron paramagnetic resonance spectroscopy in radiation research: current status and perspectives. J Pharm Bioallied Sci 2:80–87. https://doi.org/10.4103/0975-7406.67006
Samuni A, Carmichael AJ, Russo A, Mitchell JB, Riesz P (1986) On the spin trapping and ESR detection of oxygen-derived radicals generated inside cells. Proc Natl Acad Sci U S A 83:7593–7597. https://doi.org/10.1073/pnas.83.20.7593
Swartz HM, Iwasaki A, Walczak T, Demidenko E, Salikov I, Lesniewski P, Starewicz P, Schauer D, Romanyukha A (2005) Measurements of clinically significant doses of ionizing radiation using non-invasive in vivo EPR spectroscopy of teeth in situ. Appl Radiat Isot 62:293–299. https://doi.org/10.1016/j.apradiso.2004.08.016
Swartz HM, Burke G, Coey M, Demidenko E, Dong R, Grinberg O, Hilton J, Iwasaki A, Lesniewski P, Kmiec M, Lo KM, Nicolalde RJ, Ruuge A, Sakata Y, Sucheta A, Walczak T, Williams BB, Mitchell C, Romanyukha A, Schauer DA (2007) In vivo EPR for dosimetry. RadiatMeas 42:1075–1084. https://doi.org/10.1016/j.radmeas.2007.05.023
Elas M, Magwood JM, Butler B, Li C, Wardak R, DeVries R, Barth ED, Epel B, Rubinstein S, Pelizzari CA, Weichselbaum RR, Halpern HJ (2013) EPR oxygen images predict tumor control by a 50% tumor control radiation dose. Cancer Res 73:5328–5335. https://doi.org/10.1158/0008-5472.CAN-13-0069
Redler G, Elas M, Epel B, Barth ED, Halpern HJ (2013) Radiation oxygen biology with pulse electron paramagnetic resonance imaging in animal tumors. Adv Exp Med Biol 789:399–404. https://doi.org/10.1007/978-1-4614-7411-1_53
Elas M, Bell R, Hleihel D, Barth ED, McFaul C, Haney CR, Bielanska J, Pustelny K, Ahn KH, Pelizzari CA, Kocherginsky M, Halpern HJ (2008) Electron paramagnetic resonance oxygen image hypoxic fraction plus radiation dose strongly correlates with tumor cure in FSa fibrosarcomas. Int J Radiat Oncol Biol Phys 71:542–549. https://doi.org/10.1016/j.ijrobp.2008.02.022
Mulder TP, Manni JJ, Roelofs HM, Peters WH, Wiersma A (1995) Glutathione S-transferases and glutathione in human head and neck cancer. Carcinogenesis 16:619–624. https://doi.org/10.1093/carcin/16.3.619
Park JS, Yamamoto W, Sekikawa T, Matsukawa M, Okamoto R, Sasaki M, Ukon K, Tanimoto K, Kumazaki T, Nishiyama M (2002) Cellular sensitivity determinants to docetaxel in human gastrointestinal cancers. Int J Oncol 20:333–338
Prabhu K, Bhat PG, Vasudevan DM (2005) Can serum Glutathione-S-transferase levels in carcinoma cervix be a predictor of radiation response? Indian J Clin Biochem 20:95–97. https://doi.org/10.1007/BF02893050
Pamies D, Zurich MG, Hartung T (2020) Organotypic models to study human glioblastoma: studying the beast in its ecosystem. iScience 23:101633. https://doi.org/10.1016/j.isci.2020.101633
Funding
Research reported in this publication was supported by the National Institutes of Health under Award Number P20GM121322 to CPC and AB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. CPC was also supported by U54GM104942 through the NIH.
Author information
Authors and Affiliations
Contributions
CPC, AJ, and AB have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; have been involved in drafting the manuscript or revising it critically for important intellectual content; and have given final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
CPC reports personal fees from Carl Zeiss Meditec, Inc, outside of the submitted work. No other conflicts exists for the other authors.
Ethical approval
No human subjects were involved in this work. Animal use was approved by the WVU IACUC (Protocol No.1602000263.2) and includes adherence to the ethical use of laboratory animals.
Informed consent
No human subjects were involved in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cifarelli, C.P., Jacques, A. & Bobko, A. Heterogeneity of radiation response in mesenchymal subtype glioblastoma: molecular profiling and reactive oxygen species generation. J Neurooncol 152, 245–255 (2021). https://doi.org/10.1007/s11060-021-03707-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03707-9