Abstract
Glioblastoma multiforme (GBM) is an extremely malignant brain tumor for which current therapies do little to remedy. Despite aggressive treatment with surgery, radiation therapy, and chemotherapy, tumors inevitably recur as a direct consequence of the infiltrative nature of GBM. The poor prognosis of patients with GBM underscores the clear and urgent need for more precise and potent therapies. Immunotherapy is emerging as a promising means to treat GBM based on the immune system’s capacity to mediate tumor-specific cytotoxicity. In this review, we will discuss the use of peptide vaccines for the treatment of GBM. The simplicity of peptide vaccines and their ability to elicit tumor antigen-specific immune responses make them an invaluable tool for the study of brain tumor immunotherapy.
Similar content being viewed by others
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive primary malignant brain tumor affecting adults. The current standard of care for GBM includes maximal tumor resection followed by external beam radiation therapy and temozolomide (TMZ) chemotherapy. However, even with treatment GBM is invariably fatal, with a median survival of approximately 15 months [1]. Due to the imprecise nature of standard of care modalities, healthy peritumoral tissue is subject to collateral damage without complete elimination of the entire tumor cell population. Even with extremely aggressive treatment, such as the removal of an entire cerebral hemisphere [2], GBM is far too invasive to be successfully treated using these methods. Evidenced by the high rate of recurrence following standard of care management, the eradication of the entire malignant cell population is likely critical for the successful and long-term treatment of GBM, given that residual cancer stem cells are competent at repopulating new tumors [3].
In an effort to overcome the limitations of conventional therapies, immunotherapy is being rigorously tested as a means to treat GBM in light of the immune system’s capacity for molecular-guided, cell-specific cytotoxicity. Of the various immunotherapeutic modalities that could be used for the treatment of solid tumors, vaccines have garnered considerable support, in part, based on the positive track record of antiviral vaccines, a favorable safety profile, and ease of administration [4]. This review will outline peptide vaccines that are being investigated for the treatment of GBM.
A primer on peptide vaccines
A vaccine is a type of active immunotherapy that provokes the immune system into acquiring long-term immunity against an antigen of interest. Fundamentally, this activity is prompted by the administration of an immunogen in conjunction with an adjuvant—an immunological stimulator—thereby directing the activation of antigen-specific lymphocytes. Vaccines have traditionally been used in a prophylactic capacity; however, their ability to therapeutically mediate antitumor immunity is now being appreciated. Investigative cancer vaccines take many forms, including autologous/allogeneic tumor cells, tumor lysates, synthetic peptides, proteins, antigen-loaded dendritic cells, “naked” DNA, and recombinant viral vectors [4–6].
Peptide vaccines are comprised of ~8–25 amino acids that encompass an epitope within an antigenic target. To enhance their immunogenicity, these short peptides are often conjugated to a carrier protein, such as keyhole limpet hemocyanin (KLH) and tetanus toxoid. Peptide vaccines are appealing because they are relatively easy to manufacture and store, and they do not require laborious preparations that inconvenience other forms of cancer vaccines. Additionally, peptide vaccines are more chemically defined than alternative vaccine conformations, thus mitigating vaccine-to-vaccine variability [7].
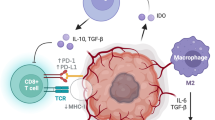
Peptide vaccines are not well suited for all immune responses. In general, secreted and extracellular antigens are targeted by humoral immunity and intracellular antigens by cell-mediated immunity, though there are exceptions. A humoral immune response is initiated though the recognition of a conformational epitope on an antigen by a B cell receptor, which promotes endocytosis and antigen processing. Conformational epitopes are native structures that arise through protein folding and are often composed of discontinuous amino acids. Short peptides do not generally mimic these conformational epitopes and are, therefore, not suitable for these purposes. Cell-mediated immunity, on the other hand, relies on presentation of short peptides in the context of major histocompatibility (MHC) (Fig. 1). Peptide vaccines are ideal for eliciting cell-mediated immunity against an antigen in which there is a known MHC-binding peptide sequence. However, due to the highly polymorphic nature of human MHC genes, not all patients will react similarly to a given epitope [7].
Immunotherapeutic targets
Tumors express an array of tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs) that can be exploited as immunotherapeutic targets. TSAs are unique to tumor cells and derive from genetic mutations or defective post-transcriptional/translational processing. TAAs are aberrantly overexpressed proteins (e.g. EGFR) or ectopically-expressed fetal-development (i.e. oncofetal), germ line-restricted (i.e. cancer/testis), and differentiation-associated proteins. TSAs arguably serve as better vaccine targets compared to TAAs because of the potential expression of TAAs on healthy cells. Immunological reactivity to normal, “self” antigens is prevented by various tolerance mechanisms, including the establishment of immunosuppressive regulatory T cells (Tregs) against “self” epitopes, the clonal deletion of “self” reactive lymphocytes, and the induction of “self” reactive lymphocytes into a state of unresponsiveness known as anergy. Consequently, the efficacy of a TAA-targeting vaccine may be compromised as a result of the deletion or suppression of TAA-cognate lymphocytes. Furthermore, because the immune system boasts potent cytotoxic potential, autoimmunity is a major concern when targeting antigens present on healthy cells. This is exemplified by studies demonstrating that experimental autoimmune encephalomyelitis can be induced through immunizations with myelin basic protein, myelin oligodendrocyte glycoprotein, or myelin proteolipid protein [8].
Peptide vaccines for GBM
GBM tumors exhibit profound genomic, transcriptomic, and proteomic alterations that may be exploited for the purposes of immunotherapy. Proteins frequently mutated or atypically expressed in GBM include EGFR, NF1, PDGFRA, PTEN, TERT, RB1, TP53, IDH1, PIK3CA and PIK3R1 [9]. Human cytomegalovirus (hCMV) antigens have also been shown to be uniquely present in GBM tumor cells [10]. Despite the tens of thousands of tumor antigens discovered in GBM [11], only a few have been pursued as targets for peptide vaccines due to the lack of conservation among GBM patients.
EGFRvIII vaccine: rindopepimut
Present in ~10–64 % of adult GBMs, EGFRvIII is a ligand-independent, constitutively active splice variant of EGFR that has been shown to enhance tumor growth and chemoresistance [9, 12, 13]. The coding sequence for EGFRvIII, residing primarily on episomal bodies, encodes a transcript devoid of exons 2-7 [14]. Consequently, a novel glycine is introduced into the amino acid sequence at the junction of exons 1 and 8 [15]. In light of its oncogenic function, tumor-specific expression, and distinctiveness from wild-type EGFR, EGFRvIII was quickly recognized as an ideal candidate for immunotherapeutic targeting.
Rindopepimut is a peptide vaccine composed of a 14mer peptide spanning the EGFRvIII-specific exon junction site conjugated to the carrier protein KLH. Initially, rindopepimut was used as an immunogen for the development of EGFRvIII-specific antibodies, which were shown to mediate effective antitumor responses against EGFRvIII-positive tumor cells when used in a passive immunotherapeutic capacity [16]. Mice vaccinated with rindopepimut, in combination with Freund’s complete adjuvant or Freund’s incomplete adjuvant plus GM-CSF, developed an EGFRvIII-specific humoral response that was capable of suppressing tumor growth and statistically enhancing median survival following intracerebral challenge with EGFRvIII-positive tumor cells. However, despite a few long term survivors, several mice succumbed to EGFRvIII-negative escape tumors [17]. This phenomenon, known as antigen escape, would later be revealed in clinical trials as one of the most critical impediments to long term effective treatment with rindopepimut.
Rindopepimut was shown to be generally well-tolerated with minimal adverse effects in a Phase I trial, known as VICTORI. In this study, newly diagnosed GBM patients were vaccinated with rindopepimut-pulsed, monocyte-derived dendritic cells. Immunological monitoring suggested that most patients developed an EGFRvIII-specific immune response [18]. To determine the safety and efficacy of rindopepimut as a peptide vaccine for patients with EGFRvIII-positive GBM, three Phase II trials were initiated. In all three trials—ACTIVATE, ACT II and ACT III—rindopepimut was administered with adjuvant GM-CSF [19–21]. ACT II and ACT III evaluated rindopepimut/GM-CSF vaccination in conjunction with TMZ maintenance therapy based on earlier reports showing that TMZ-induced lymphopenia enhanced immunotherapeutic efficacy [22, 23]. The results from these trials further confirmed the safety of rindopepimut and demonstrated a statistical increase in median progression-free (PFS) and overall survival (OS) in vaccinated patients, compared to a standard of care treated cohort (PFS = 6.4 months, OS = 15.2 months) [19–21]. Consistent with histological data from preclinical investigations, most patients unfortunately succumbed to recurrent tumors that were devoid of EGFRvIII expression. (See Table 1 for more information on rindopepimut clinical trials).
Under the auspices of Celldex Therapeutics, Inc., enrollment is currently open for two rindopepimut clinical trials: ReACT and ACT IV. ReACT is a non-pivotal Phase II trial for patients with recurrent GBM, and will include a group of relapsed patients that is refractory to treatment with bevacizumab (anti-VEGF monoclonal antibody). In addition to receiving bevacizumab, patients will be vaccinated with either rindopepimut/GM-CSF or KLH. ACT IV is a Phase III trial that will be conducted at over 200 locations world-wide. Patients will receive either rindopepimut/GM-CSF or KLH, as well as TMZ maintenance therapy at the standard dose.
IDH1 R132H vaccine
Isocitrate dehydrogenase 1 (IDH1) is a cytosolic enzyme that is frequently mutated in gliomas [24]. The IDH1 R132H mutation, present in 5–12 % of GBMs, is typically associated with secondary GBMs that affect young adults [9, 24]. The R132H amino acid substitution alters the catalytic site, preventing IDH1 from catalyzing the NADP+ dependent oxidative decarboxylation of isocitrate to α-ketoglutarate (αKG) [25]. Alternatively, IDH1 R132H consumes NADPH and αKG to produce the oncometabolite 2-hydroxyglutrate (2HG) [25], which has been shown to perturb protein and DNA methylation [26]. Wild-type IDH1 also performs NADPH/CO2-dependent reductive carboxylation of αKG to isocitrate (reverse reaction)—a reaction that is implicated in fatty acid and cholesterol biosynthetic pathways [27]. Lipogenic dysregulation promoted by the inability of IDH1 R132H to execute this function may underlie IDH1 R132H’s association with less aggressive, secondary GBMs [27, 28].
A recent preclinical study demonstrated that vaccination of MHC-humanized (i.e. HLA-A*0201 HLA-DRA*0101 HLA-DRB1*0101) mice with a peptide vaccine representing amino acids 123-142 (p123-142) of IDH1 R132H was capable of suppressing the growth of an IDH1 R132H-positive sarcoma. Consistent with the identification of MHC class II epitopes within p123-142, p123-142 vaccinated mice possessed IDH1 R132H-reactive, IFN-γ-producing CD4 + T cells. Supporting the contribution of this arm of immunity, CD4 depletion diminished vaccine-mediated tumor suppression [29]. Though these results are encouraging, further studies are needed to determine whether an IDH1 R132H vaccine is effective in the context of a glioma, which do not generally express MHC II [30].
CMV vaccine: PEP-CMV
Human cytomegalovirus (hCMV) is a common herpes virus that causes life threatening disease in infants and immunocompromised individuals. Approximately 50–80 % of healthy individuals have been exposed to CMV, though primary infection is generally asymptomatic. Once the immune system suppresses the initial infection, CMV becomes latent, commonly using myeloid cells as a reservoir [31].
Though their role and origin remains controversial, several hCMV proteins have been detected in GBM specimens and not in normal tissues [32]. These proteins include IE1, US28, pp65, gB, HCMV IL-10, and pp28 [32]. Due to their exclusivity in tumor cells, these antigens have been exploited as immunotherapeutic targets. A peptide cocktail containing class I and class II restricted epitopes from CMV antigens, known as PEP-CMV, will soon be investigated in a clinical trial known as PERFORMANCE.
Multiple GBM antigen vaccines
Due to the high rate of relapse encountered by vaccination against a single tumor antigen, multiple tumor antigen-targeting vaccines are likely required to combat the vast antigenic heterogeneity present among cells within a tumor population. The German company immatics Biotechnologies GmbH is currently investigating an 11 peptide GBM vaccine—known as IMA950—that targets multiple high frequency tumor antigens, which were revealed through mass spectrometric analysis of MHC-complexed peptides from 30 primary human GBM specimens. Nine of the peptides bind to the common MHC I allele HLA-A*02, and two of the peptides bind to various HLA-DR (MHC II) alleles [33]. A Phase I trial conducted in the UK recently met two primary endpoints for safety and immunogenicity, demonstrating that ~90 % of the patients responded to the vaccine. An additional multiple tumor antigen-targeting GBM vaccine, known as SL-701 by Stemline Therapeutics Inc., will soon begin Phase II testing based on the recent acceptance of an IND.
Immunopotentiation using adjuvants
Adjuvants are compounds that enhance immunogenicity. The delivery of an antigen in the absence of an adjuvant generally leads to tolerance; therefore, adjuvants are an essential component of vaccine strategies. Peptide vaccines for glioblastoma, such as rindopepimut and PEP-CMV, are typically resuspended in water or saline and delivered with the adjuvant GM-CSF, which has been shown to recruit and activate antigen presenting cells [34]. One adjuvant that is showing great promise with regard to cancer vaccines is dendritic cells—dubbed “nature’s adjuvant.” The utility of dendritic cells as an adjuvant derives from their capacity to provide the costimulation and immunostimulatory molecules needed to prime naïve T cells. Numerous studies have shown that autologous dendritic cells pulsed with peptides or tumor lysate are capable of inducing powerful antitumor responses and are well tolerated in human subjects (reviewed in [35]). Furthermore, because many traditional adjuvants exert their effects on dendritic cells, dendritic cells may be treated with adjuvants ex vivo prior to vaccination, thereby minimizing the risk of toxicity associated with the direct delivery of adjuvants into a patient. Because adjuvants play an integral role in the immune response, meeting the demand for effective, yet safe, adjuvants will be necessary for the progression of cancer vaccines as a feasible treatment strategy.
Overcoming the challenges of tumor vaccines
Though cancer vaccines are demonstrably safe and effective, studies have shown that they are rarely curative. GBM tumors have evolved certain mechanisms that allow them to evade immunological attack, and these defense mechanisms, by their very nature, impede the effectiveness of cancer vaccines. For example, GBM cancer stem cells and migrating glioma cells have been shown to lack expression of MHC molecules [36, 37]. This behavior precludes antigen presentation and prevents tumor antigen-cognate lymphocytes from recognizing tumor cells in an MHC-dependent manner. GBM tumors also mediate profound immunosuppression. GBM-associated cancer stem cells produce an arsenal of immunosuppressive molecules including PD-L1, an inhibitor of T cell proliferation, and the Treg-inducing cytokine TGF-β [38]. Furthermore, host-induced immunosuppression at the GBM tumor microenvironment is coordinated by regulatory T cells (Tregs), myeloid-derived suppressor cells, and type 2 microglia. These cells have been shown to repress effector T cell activity through direct cell-to-cell contact or by secreting immunosuppressive mediators [38, 39]. The contribution of these mechanisms to immunotherapeutic recalcitrance has been demonstrated by studies showing that their inhibition or manipulation leads to more effective antitumor immunity [40–42]. However, uncovering clinically applicable modalities that safely, specifically, and potently target these mechanisms remains a challenge.
Current tumor vaccines are also encumbered by the vast antigenic heterogeneity displayed among tumor cells. Both EGFRvIII and IDH1 R132H frequently exhibit heterogeneous expression within GBM tumors [43, 44]. Consequently, vaccination strategies targeting a single tumor antigen commonly lead to the outgrowth of antigen-loss escape variants, exemplified in clinical trials with rindopepimut. Due to the effort and cost associated with vaccine development and clinical evaluation, peptide vaccines are generally only practical for targeting common tumor antigens, and exhaustive molecular examination of numerous GBM specimens has revealed that only a few immunotherapeutically-feasible tumor antigens are widespread and conserved [11, 45]. To cope with the unique antigenic landscape of an individual tumor, dendritic cells—the most potent antigen presenting cells—offer a powerful platform for creating personalized and multivalent vaccines tailored to an individual patient’s tumor. Methods for loading dendritic cells with tumor antigen include total tumor RNA transfection, fusion to tumor cells, and pulsation with apoptotic tumor cells, tumor lysate, or synthetic peptides. Several dendritic cell vaccines for the treatment of GBM are currently under investigation in clinical trials and have been reviewed elsewhere [6, 46, 47].
Conclusion
GBM is a devastating disease, for which very few treatment options exist. Therapies that are traditionally used to treat GBM are invasive, damaging to healthy tissue, and do not provide long term relief; therefore, there is a clear and urgent need for safer, more selective antitumor modalities. Immunotherapy is emerging as a promising means to treat malignant brain tumors. Particularly, cancer vaccines are proving to be a minimally-invasive immunotherapeutic strategy that is safe, selective, and sympathetic towards the delicate nature of the central nervous system. The use of peptide vaccines to target individual tumor antigens is a logical approach to easily and effectively elicit antitumor immunity; however, the resilience of cancer is reaffirmed by the high incidence of antigen escape that generally follows vaccination. Future cancer vaccines will likely employ a personalized approach to target a vast array of unique tumor antigens—a seemingly overwhelming task for a peptide-based approach. Despite the apparent limitations of peptide vaccines, they remain an essential tool for the elucidation of brain tumor immunotherapy.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, 9 Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Dandy WE (1928) Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA 90:823–825
Liu Q, Nguyen DH, Dong Q, Shitaku P, Chung K, Liu OY, Tso JL, Liu JY, Konkankit V, Cloughesy TF, Mischel PS, Lane TF, Liau LM, Nelson SF, Tso CL (2009) Molecular properties of CD133 + glioblastoma stem cells derived from treatment-refractory recurrent brain tumors. J Neurooncol 94:1–19. doi:10.1007/s11060-009-9919-z
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10:909–915. doi:10.1038/nm1100
Evel-Kabler K, Chen SY (2006) Dendritic cell-based tumor vaccines and antigen presentation attenuators. Mol Ther 13:850–858. doi:10.1016/j.ymthe.2006.02.009
Xu LW, Chow KK, Lim M, Li G (2014) Current vaccine trials in glioblastoma: a review. J Immunol Res 2014:796856. doi:10.1155/2014/796856
Purcell AW, McCluskey J, Rossjohn J (2007) More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov 6:404–414. doi:10.1038/nrd2224
Leadbetter EA, Bourque CR, Devaux B, Olson CD, Sunshine GH, Hirani S, Wallner BP, Smilek DE, Happ MP (1998) Experimental autoimmune encephalomyelitis induced with a combination of myelin basic protein and myelin oligodendrocyte glycoprotein is ameliorated by administration of a single myelin basic protein peptide. J Immunol 161:504–512
Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, Hawkins C, Majewski J, Jones C, Costello JF, Iavarone A, Aldape K, Brennan CW, Jabado N, Pfister SM (2014) Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer 14:92–107. doi:10.1038/nrc3655
Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ (2002) Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62:3347–3350
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. doi:10.1016/j.cell.2013.09.034
Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, Furnari FB, White FM (2007) Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci USA 104:12867–12872. doi:10.1073/pnas.0705158104
Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS, Bigner DD (1990) Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res 50:8017–8022
Vogt N, Lefevre SH, Apiou F, Dutrillaux AM, Cor A, Leuraud P, Poupon MF, Dutrillaux B, Debatisse M, Malfoy B (2004) Molecular structure of double-minute chromosomes bearing amplified copies of the epidermal growth factor receptor gene in gliomas. Proc Natl Acad Sci USA 101:11368–11373. doi:10.1073/pnas.0402979101
Wikstrand CJ, Reist CJ, Archer GE, Zalutsky MR, Bigner DD (1998) The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J Neurovirol 4:148–158. doi:10.3109/13550289809114515
Sampson JH, Crotty LE, Lee S, Archer GE, Ashley DM, Wikstrand CJ, Hale LP, Small C, Dranoff G, Friedman AH, Friedman HS, Bigner DD (2000) Unarmed, tumor-specific monoclonal antibody effectively treats brain tumors. Proc Natl Acad Sci USA 97:7503–7508. doi:10.1073/pnas.130166597
Heimberger AB, Crotty LE, Archer GE, Hess KR, Wikstrand CJ, Friedman AH, Friedman HS, Bigner DD, Sampson JH (2003) Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res 9:4247–4254
Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE 2nd, Lally-Goss D, McGehee-Norman S, Paolino A, Reardon DA, Friedman AH, Friedman HS, Bigner DD (2009) An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther 8:2773–2779. doi:10.1158/1535-7163.MCT-09-0124
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE 2nd, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729. doi:10.1200/JCO.2010.28.6963
Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling R, Shi W, Vredenburgh JJ, Bigner DD, Heimberger AB (2011) Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol 13:324–333. doi:10.1093/neuonc/noq157
Lai RK, Recht LD, Reardon DA, Paleologos N, Groves M, Rosenfeld MR, Davis T, Archer G, Green J, Heimberger A, Sampson JH (2011) Long-term follow-up of ACT III: A Phase II trial of Rindopepimut (CDX-110) in newly diagnosed glioblastoma. Proceedings of the 16th Annual Scientific Meeting of the Society for Neuro-Oncology in Conjunction with the AANS/CNS Section on Tumors: 34–40
Sanchez-Perez LA, Choi BD, Archer GE, Cui X, Flores C, Johnson LA, Schmittling RJ, Snyder D, Herndon JE 2nd, Bigner DD, Mitchell DA, Sampson JH (2013) Myeloablative temozolomide enhances CD8(+) T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS ONE 8:e59082. doi:10.1371/journal.pone.0059082
Mitchell DA, Cui X, Schmittling RJ, Sanchez-Perez L, Snyder DJ, Congdon KL, Archer GE, Desjardins A, Friedman AH, Friedman HS, Herndon JE 2nd, McLendon RE, Reardon DA, Vredenburgh JJ, Bigner DD, Sampson JH (2011) Monoclonal antibody blockade of IL-2 receptor alpha during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood 118:3003–3012. doi:10.1182/blood-2011-02-334565
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. doi:10.1056/NEJMoa0808710
Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, Rabinowitz JD, Carroll M, Su SM, Sharp KA, Levine RL, Thompson CB (2010) The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17:225–234. doi:10.1016/j.ccr.2010.01.020
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19:17–30. doi:10.1016/j.ccr.2010.12.014
Leonardi R, Subramanian C, Jackowski S, Rock CO (2012) Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J Biol Chem 287:14615–14620. doi:10.1074/jbc.C112.353946
Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ (2012) Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481:385–388. doi:10.1038/nature10642
Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, Keil M, Balss J, Rauschenbach K, Grabowska AK, Vogler I, Diekmann J, Trautwein N, Eichmuller SB, Okun J, Stevanovic S, Riemer AB, Sahin U, Friese MA, Beckhove P, von Deimling A, Wick W, Platten M (2014) A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. doi:10.1038/nature13387
Takamura Y, Ikeda H, Kanaseki T, Toyota M, Tokino T, Imai K, Houkin K, Sato N (2004) Regulation of MHC class II expression in glioma cells by class II transactivator (CIITA). Glia 45:392–405. doi:10.1002/glia.10343
Dey M, Ahmed AU, Lesniak MS (2014) Cytomegalovirus and glioma: putting the cart before the horse. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp-2014-307727
Dziurzynski K, Chang SM, Heimberger AB, Kalejta RF, McGregor Dallas SR, Smit M, Soroceanu L, Cobbs CS, Hcmv, Gliomas S (2012) Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol 14: 246–255. doi:10.1093/neuonc/nor227
James A, Mulholland P, Peoples S, Al-Salihi O, Twelves C, Jefferies S, McBain C, Schoor O, Hilf N, Lindner J, Kutscher S, Peters J, Halford S, McGuigan L, Ritchie J, Singh-Jasuja H, Rampling R (2012) Updated results from a Cancer Research UK first in man Phase I trial of IMA950 (a novel multi peptide vaccine) plus GM-CSF in patients with newly diagnosed glioblastoma. European Society for Medical Oncology. Vienna, Austria
Heufler C, Koch F, Schuler G (1988) Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med 167:700–705
Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ (2013) Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev 39:891–907. doi:10.1016/j.ctrv.2013.05.007
Zagzag D, Salnikow K, Chiriboga L, Yee H, Lan L, Ali MA, Garcia R, Demaria S, Newcomb EW (2005) Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab Investig 85:328–341. doi:10.1038/labinvest.3700233
Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, Mortini P, Ferrone S, Doglioni C, Marincola FM, Galli R, Parmiani G, Maccalli C (2010) Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res 16:800–813. doi:10.1158/1078-0432.CCR-09-2730
Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Sawaya R, Lang FF, Heimberger AB (2010) Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res 16:461–473. doi:10.1158/1078-0432.CCR-09-1983
Kershaw MH, Devaud C, John LB, Westwood JA, Darcy PK (2013) Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. Oncoimmunology 2:e25962. doi:10.4161/onci.25962
Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim C, Tobias AL, Cheng Y, Kim J, Zhang L, Qiao J, Han Y, Lesniak MS (2014) Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res. doi:10.1158/1078-0432.CCR-14-0514
Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM (2005) Gemcitabine selectively eliminates splenic Gr-1 +/CD11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11:6713–6721. doi:10.1158/1078-0432.CCR-05-0883
Sampson JH, Schmittling RJ, Archer GE, Congdon KL, Nair SK, Reap EA, Desjardins A, Friedman AH, Friedman HS, Herndon JE 2nd, Coan A, McLendon RE, Reardon DA, Vredenburgh JJ, Bigner DD, Mitchell DA (2012) A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS ONE 7:e31046. doi:10.1371/journal.pone.0031046
Del Vecchio CA, Giacomini CP, Vogel H, Jensen KC, Florio T, Merlo A, Pollack JR, Wong AJ (2013) EGFRvIII gene rearrangement is an early event in glioblastoma tumorigenesis and expression defines a hierarchy modulated by epigenetic mechanisms. Oncogene 32:2670–2681. doi:10.1038/onc.2012.280
Agarwal S, Sharma MC, Jha P, Pathak P, Suri V, Sarkar C, Chosdol K, Suri A, Kale SS, Mahapatra AK, Jha P (2013) Comparative study of IDH1 mutations in gliomas by immunohistochemistry and DNA sequencing. Neuro Oncol 15:718–726. doi:10.1093/neuonc/not015
Cancer Genome Atlas Research N (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068. doi:10.1038/nature07385
Jackson C, Ruzevick J, Brem H, Lim M (2013) Vaccine strategies for glioblastoma: progress and future directions. Immunotherapy 5:155–167. doi:10.2217/imt.12.155
Aguilar LK, Arvizu M, Aguilar-Cordova E, Chiocca EA (2012) The spectrum of vaccine therapies for patients with glioblastoma multiforme. Curr Treat Options Oncol 13:437–450. doi:10.1007/s11864-012-0208-2
Conflict of interest
Employment or Leadership Position: None; Consultant or Advisory Role: John H. Sampson, Celldex Therapeutics (C); Stock Ownership: None Honoraria: None; Research Funding: John H. Sampson, Celldex Therapeutics; Expert Testimony: None Other Remuneration: John H. Sampson receives funding under the Duke University Faculty Plan from license fees paid to Duke University by Celldex Therapeutics. Duke University also has the potential to receive patent-related royalties.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swartz, A.M., Batich, K.A., Fecci, P.E. et al. Peptide vaccines for the treatment of glioblastoma. J Neurooncol 123, 433–440 (2015). https://doi.org/10.1007/s11060-014-1676-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1676-y