Abstract
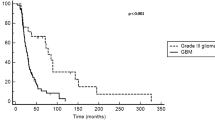
Bevacizumab is one of the rare drugs that could improve high-grade glioma outcome after failure of chemoradiotherapy. However, to date, there is no biomarker predictive for efficacy of bevacizumab therapy in terms of survival improvement for patients with high-grade glioma. We performed a retrospective analysis of clinical factors associated with patient survival using a training cohort of 110 consecutive patients treated with bevacizumab for recurrent high-grade glioma and an independent validation cohort of 109 patients. In the training cohort, 110 consecutive patients received bevacizumab-based therapy. The number of chemotherapy cycles delivered was 1,411. Median follow-up was 12 months. Thirty-four patients (31%) had objective partial response and 24% had stable disease on magnetic resonance imaging evaluation. Median progression-free survival (PFS) and overall survival (OS) were 4.3 and 9.2 months, respectively. On univariate analysis, among classical prognosis factors, only Karnofsky status ≥70% was associated with improved outcome. Surprisingly, patients with low bevacizumab dose intensity (<5 mg/kg/week) had better PFS (12 vs. 2 months, P < 0.0001) and OS (16 vs. 6 months, P = 0.0002). On multivariate analysis, low bevacizumab dose intensity was the most significant independent prognostic factor of survival. Analysis of the validation cohort yielded similar results, externally validating this observation. This large retrospective study using two independent cohorts of high-grade glioma suggests that the currently recommended dosage of bevacizumab (5 mg/kg/week) is not optimal. Further prospective randomized trials using lower dosages are warranted.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Lamborn KR, Yung WK, Chang SM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10:162–170
Ballman KV, Buckner JC, Brown PD et al (2007) The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol 9:29–38
Yung WK, Albright RE, Olson J et al (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83:588–593
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Gupta K, Radotra BD, Banerjee AK, Nijhawan R (2004) Quantitation of angiogenesis and its correlation with vascular endothelial growth factor expression in astrocytic tumors. Anal Quant Cytol Histol 26:223–229
Hong YK, Lange-Asschenfeldt B, Velasco P et al (2004) VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. Faseb J 18:1111–1113
Nam DH, Park K, Suh YL, Kim JH (2004) Expression of VEGF and brain specific angiogenesis inhibitor-1 in glioblastoma: prognostic significance. Oncol Rep 11:863–869
Takano S, Tsuboi K, Matsumura A, Nose T (2003) Anti-vascular endothelial growth factor antibody and nimustine as combined therapy: effects on tumour growth and angiogenesis in human glioblastoma xenografts. Neuro Oncol 5:1–7
Sathornsumetee S, Cao Y, Marcello JE et al (2008) Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol 26:271–278
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Miller K, Wang M, Gralow J et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
Escudier B, Bellmunt J, Negrier S et al (2010) Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 28:2144–2150
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A (1994) Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature 367:576–579
Folkman J (1985) Tumor angiogenesis. Adv Cancer Res 43:175–203
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Parangi S, O’Reilly M, Christofori G et al (1996) Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci USA 93:2002–2007
Vredenburgh JJ, Desjardins A, Herndon JE II et al (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259
Norden AD, Drappatz J, Muzikansky A et al (2009) An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol 92:149–155
Xu T, Chen J, Lu Y, Wolff JE (2010) Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis. BMC Cancer 10:252
de Groot JF, Fuller G, Kumar AJ et al (2010) Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol 12:233–242
Keunen O, Johansson M, Oudin A et al (2011) Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA
Lucio-Eterovic AK, Piao Y, de Groot JF (2009) Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res 15:4589–4599
de Groot JF (2011) High-dose antiangiogenic therapy for glioblastoma: less may be more? Clin Cancer Res
Kabbinavar F, Hurwitz HI, Fehrenbacher L et al (2003) Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60–65
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
V. Lorgis, G. Maura, S. Ladoire, and F. Ghiringhelli share coauthorship.
Rights and permissions
About this article
Cite this article
Lorgis, V., Maura, G., Coppa, G. et al. Relation between bevacizumab dose intensity and high-grade glioma survival: a retrospective study in two large cohorts. J Neurooncol 107, 351–358 (2012). https://doi.org/10.1007/s11060-011-0748-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0748-5
Keywords
Profiles
- Lionel Apetoh View author profile