Abstract
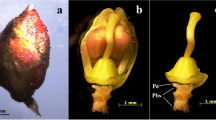
To propagate Dendrocalamus latiflorus, we used in vivo inflorescences to produce calli on Murashige and Skoog basal (MS) medium supplemented with 3 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D), 2 mg/l kinetin, 250 mg/l polyvinyl pyrrolidone (PVP), and 1% coconut milk. Multiple shoots were generated on MS medium supplemented with 0.1 mg/l thidiazuron (TDZ). The green plantlets were successfully transferred to soil. Multiple albino shoots also regenerated and were able to proliferate on medium containing cytokinins, especially TDZ. Albino multiple shoots rooted in medium containing α-naphthaleneacetic acid (NAA), and callus formation was observed in the presence of 2,4-D and picloram. Green and albino regenerates flowered after 8 months of subculture. The flowering ratio increased to 44% after three treatments in medium containing 1 mg/l TDZ. Morphological observations revealed that the in vitro green and albino flower organs were normal. However, pollen derived from the in vitro flowers of both the green and albino plants were sterile.

Similar content being viewed by others
Abbreviations
- 2,4-D :
-
2,4-dichlorophenoxyacetic acid
- BA :
-
N6-benzyladenine
- IBA :
-
indolebutyric acid
- 2-ip :
-
2-isopentyl adenine
- MS :
-
Murashige and Skoog medium
- NAA :
-
α-naphthaleneacetic acid
- PVP :
-
polyvinyl pyrrolidone
- TDZ :
-
thidiazuron
References
Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44:117–122
Chambers SM, Heuch JH, Pirrie A (1991) A Micropropagation and in vitro flowering of bamboo Dendrocalamus hamiltonii Munro. Plant Cell Tiss Organ Cult 26:45–48
Chang WC (1991) Bamboo. In: Bajaja YPS (ed) Biotechnology in agriculture and forestry, vol 15. Tree III. Springer, New York, pp 221–237
Chang WC (1994) Somatic embryogenesis of Bambusa oldhamii, Bambsa beecheyana and Sinocalamus latiflora. In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woods plants. Kluwer Academic Publishers, Dordrecht, pp 53–65
Chang WC, Ho CW (1997) Micropropagation of bamboo. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 39. Hightech and micropropagation. Springer, New York, pp 203–219
Chaturvedi HC, Sharma M, Sharma AK (1993) In vitro regeneration of Dendrocalamus strictus Nees through nodal segment taken from field-grown culms. Plant Sci 91:97–101
Day A, Ellis THN (1984) Chloroplast DNA deletions associated with wheat plant regenerated from pollen: possible basis for maternal inheritance of chloroplasts. Cell 39:359–368
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Dunford R, Walden RM (1991) Plastid genome structure and plastid-related transcript levels in albino barley plants derived from anther culture. Curr Genet 20:339–347
Gielis J, Peeters H, Gillis K, Oprins J, Debergh PC (2001) Tissue culture strategies for genetic improvement of bamboo. Acta Hortic 552:195–203
Harada T, Sato T, Asaka D, Matsukawa I (1991) Large-scale deletions of rice plastid DNA in anther culture. Theor Appl Genet 81:157–161
Harada T, Ishikawa R, Niizeki M, Saito KI (1992) Pollen-derived rice calli that have large deletions in plastid DNA do not require protein synthesis in plastids for growth. Mol Gen Genet 233:145–150
Ho CW, Chang WC (1998) In vitro flowering of albino bamboo (Bambusa oldhamii Munro.) regenerants derived from an eleven-year-old embryogenic cell line. Acta Hortic 461:433–438
Hsu YH, Annamalai AP, Lin CS, Chen YY, Chang WC, Lin NS (2000) A sensitive method for detecting bamboo mosaic virus (BaMV) and establishment of BaMV-free meristem tip cultures. Plant Pathol 49:101–107
Hu CY, Wang PJ (1983) Meristem, shoot tip, and bud culture. In: Evans DA, Sharp WR, Ammirato PV, Yamada Y (eds) Handbook of plant cell culture, vol 1 Techniques for propagation and breeding. Macmillan, New York, pp 177–227
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Kawata M, Harada T, Shimamoto Y, Oono K, Takaiwa F (1997) Short inverted repeats function as hotspots of intermolecular recombination giving rise to oligomers of deleted plastid DNAs (ptDNAs). Curr Genet 31:179–184
Lin CS, Chang WC (1998) Micropropagation of Bambusa edulis through nodal explants of field-grown clums and flowering of regenerated plantlets. Plant Cell Rep 17:617–620
Lin CS, Lin CC, Chang WC (2003) In vitro flowering of Bambusa edulis and subsequent plantlet survival. Plant Cell Tissue Organ Cult 72:71–78
Lin CS, Lin CC, Chang WC (2004) Effect of thidiazuron on vegetative tissue-derived somatic embryogenesis and flowering of Bambusa edulis. Plant Cell Tissue Organ Cult 76:75–82
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Nadgauda RS, Parasharami VA, Mascarenhas AF (1990) Precocious flowering and seeding behaviour in tissue-cultured bamboos. Nature 344:335–336
Nadgir AL, Phadke CH, Gupta PK, Parsharan VA, Nair S, Mascarenhas AF (1984) Rapid multiplication of bamboo by tissue culture. Silvae Genet 33:219–233
Prutpongse P, Gavinlertvatana P (1992) In vitro micropropagation of 54 species from 15 genera of bamboo. HortScience 27:453–454
Ramanayake SMSD, Wanniarachchi WAVR, Tennakoon TMA (2001) Axillary shoot proliferation and in vitro flowering in an adult giant bamboo, Dendrocalamus giganteus Wall. Ex Munro. In Vitro Cell Dev Biol-Plant 37:661–667
Rout GR, Das P (1994) Somatic embryogenesis and in vitro flowering of 3 species of bamboo. Plant Cell Rep 13:683–686
Scorza R (1982) In vitro flowering. Hortic Rev 4:106–127
Singh M, Jaiswal U, Jaiswal VS (2000) Thidiazuron-induced in vitro flowering in Dendrocalamus strictus Nees. Curr Sci 79:1529–1530
Yeh ML, Chang WC (1987) Plant regeneration via somatic embryogenesis in mature embryo-derived callus culture of Sinocalamus latiflora (Munro) McClure. Plant Sci 51:93–96
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lin, CS., Liang, C.J., Hsaio, H.W. et al. In vitro flowering of green and albino Dendrocalamus latiflorus . New Forests 34, 177–186 (2007). https://doi.org/10.1007/s11056-007-9045-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-007-9045-8