Abstract
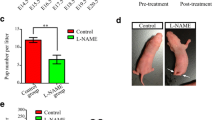
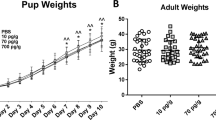
It is known that preeclampsia alters the neurodevelopment of the descent of preeclamptic mothers. Given that cerebellar development occurs mainly when the brain in gestation is impacted by the preeclampsia, and it is described that in preeclampsia exist a placental dysregulation of pro- and anti-angiogenic factors, such as vascular endothelial growth factor and its main receptors: VEGF receptor 2 phosphorylated at Y951 and VEGF receptor 2 phosphorylated at Y1172, we decided to evaluate, the effect of experimental preeclampsia on the cerebellar expression of VEGF and two of its receptors, pY951 VEGF R2 and pY1172 VEGF R2. This evaluation was carried out in the progeny of preeclamptic mothers in an ontogenetic stage equivalent to infancy, using the experimental model of preeclampsia based on the administration of N (ω)-nitro-L-arginine methyl ester in pregnant Sprague–Dawley rats. As part of our assessment of the functional correlate of the cerebellum, we evaluated the balance and locomotor learning in the progeny using the rotarod test. We observed that experimental preeclampsia increased the blood pressure in the mothers, along with proteinuria and the alteration of the level of vascular endothelial growth factor in the cerebellar vermis of both sexes progeny. Furthermore, it was observed an increase VEGF receptor 2 phosphorylated at Y951 in the male progeny. Additionally, it was observed a decrease in locomotor learning among daughters of preeclamptic mothers. Future studies are necessary to continue understanding the complex pathophysiology of preeclampsia and how it affects the neurodevelopment of the offspring.









Similar content being viewed by others
References
Ahmad, S., Hewett, P. W., Al-Ani, B., Sissaoui, S., Fujisawa, T., Cudmore, M. J., & Ahmed, A. (2011). Autocrine activity of soluble Flt-1 controls endothelial cell function and angiogenesis. Vascular cell, 3(1), 15. https://doi.org/10.1186/2045-824X-3-15
Aldinger, K. A., Thomson, Z., Phelps, I. G., Haldipur, P., Deng, M., Timms, A. E., Hirano, M., Santpere, G., Roco, C., Rosenberg, A. B., Lorente-Galdos, B., Gulden, F. O., O'Day, D., Overman, L. M., Lisgo, S. N., Alexandre, P., Sestan, N., Doherty, D., Dobyns, W. B., Seelig, G., … Millen, K. J. (2021). Spatial and cell type transcriptional landscape of human cerebellar development. Nature neuroscience, 24(8), 1163–1175. https://doi.org/10.1038/s41593-021-00872-y
Autiero, M., Waltenberger, J., Communi, D., Kranz, A., Moons, L., Lambrechts, D., Kroll, J., Plaisance, S., De Mol, M., Bono, F., Kliche, S., Fellbrich, G., Ballmer-Hofer, K., Maglione, D., Mayr-Beyrle, U., Dewerchin, M., Dombrowski, S., Stanimirovic, D., Van Hummelen, P., Dehio, C., … Carmeliet, P. (2003). Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nature medicine, 9(7), 936–943. https://doi.org/10.1038/nm884
Badagionis, M., Sergentanis, T. N., Pervanidou, P., Kalampokas, E., Vlahos, N., & Eleftheriades, M. (2022). Preeclampsia and Cerebral Palsy in Offspring. Children (Basel, Switzerland), 9(3), 385. https://doi.org/10.3390/children9030385
Bale T. L. (2016). The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues in clinical neuroscience, 18(4), 459–464. https://doi.org/10.31887/DCNS.2016.18.4/tbale
Bergman, L., Acurio, J., Leon, J., Gatu, E., Friis, T., Nelander, M., Wikström, J., Larsson, A., Lara, E., Aguayo, C., Torres-Vergara, P., Wikström, A. K., & Escudero, C. (2021). Preeclampsia and Increased Permeability Over the Blood-Brain Barrier: A Role of Vascular Endothelial Growth Receptor 2. American journal of hypertension, 34(1), 73–81. https://doi.org/10.1093/ajh/hpaa142
Bergman, L., Hastie, R., Bokström-Rees, E., Zetterberg, H., Blennow, K., Schell, S., Imberg, H., Langenegger, E., Moodley, A., Walker, S., Tong, S., & Cluver, C. (2022). Cerebral biomarkers in neurologic complications of preeclampsia. American journal of obstetrics and gynecology, 227(2), 298.e1–298.e10. https://doi.org/10.1016/j.ajog.2022.02.036
Bohlen, M., Cameron, A., Metten, P., Crabbe, J. C., & Wahlsten, D. (2009). Calibration of rotational acceleration for the rotarod test of rodent motor coordination. Journal of neuroscience methods, 178(1), 10–14. https://doi.org/10.1016/j.jneumeth.2008.11.001
Bokslag, A., van Weissenbruch, M., Mol, B. W., & de Groot, C. J. (2016). Preeclampsia; short and long-term consequences for mother and neonate. Early human development, 102, 47–50. https://doi.org/10.1016/j.earlhumdev.2016.09.007
Carmeliet, P., & Ruiz de Almodovar, C. (2013). VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cellular and molecular life sciences : CMLS, 70(10), 1763–1778. https://doi.org/10.1007/s00018-013-1283-7
Carver, A. R., Tamayo, E., Perez-Polo, J. R., Saade, G. R., Hankins, G. D., & Costantine, M. M. (2014). The effect of maternal pravastatin therapy on adverse sensorimotor outcomes of the offspring in a murine model of preeclampsia. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience, 33, 33–40. https://doi.org/10.1016/j.ijdevneu.2013.11.004
Dachew, B. A., Mamun, A., Maravilla, J. C., & Alati, R. (2018a). Association between hypertensive disorders of pregnancy and the development of offspring mental and behavioural problems: A systematic review and meta-analysis. Psychiatry research, 260, 458–467. https://doi.org/10.1016/j.psychres.2017.12.027
Dachew, B. A., Mamun, A., Maravilla, J. C., & Alati, R. (2018b). Pre-eclampsia and the risk of autism-spectrum disorder in offspring: meta-analysis. The British journal of psychiatry : the journal of mental science, 212(3), 142–147. https://doi.org/10.1192/bjp.2017.27
Dachew, B. A., Scott, J. G., Betts, K., Mamun, A., & Alati, R. (2020). Hypertensive disorders of pregnancy and the risk of offspring depression in childhood: Findings from the Avon Longitudinal Study of Parents and Children. Development and psychopathology, 32(3), 845–851. https://doi.org/10.1017/S0954579419000944
Dachew, B. A., Scott, J. G., Mamun, A., & Alati, R. (2019a). Pre-eclampsia and the risk of attention-deficit/hyperactivity disorder in offspring: Findings from the ALSPAC birth cohort study. Psychiatry research, 272, 392–397. https://doi.org/10.1016/j.psychres.2018.12.123
Dachew, B. A., Scott, J. G., Mamun, A., & Alati, R. (2019b). Hypertensive disorders of pregnancy and the risk of anxiety disorders in adolescence: Findings from the Avon Longitudinal Study of Parents and Children. Journal of psychiatric research, 110, 159–165. https://doi.org/10.1016/j.jpsychires.2019.01.001
de Alwis, N., Binder, N. K., Beard, S., Mangwiro, Y. T., Kadife, E., Cuffe, J. S., Keenan, E., Fato, B. R., Kaitu'u-Lino, T. J., Brownfoot, F. C., Marshall, S. A., & Hannan, N. J. (2022). The L-NAME mouse model of preeclampsia and impact to long-term maternal cardiovascular health. Life science alliance, 5(12), e202201517. https://doi.org/10.26508/lsa.202201517
Ehrenstein, V., Rothman, K. J., Pedersen, L., Hatch, E. E., & Sørensen, H. T. (2009). Pregnancy-associated hypertensive disorders and adult cognitive function among Danish conscripts. American journal of epidemiology, 170(8), 1025–1031. https://doi.org/10.1093/aje/kwp223
Elkafrawi, D., Sisti, G., Araji, S., Khoury, A., Miller, J., & Rodriguez Echevarria, B. (2020). Risk Factors for Neonatal/Maternal Morbidity and Mortality in African American Women with Placental Abruption. Medicina (Kaunas, Lithuania), 56(4), 174. https://doi.org/10.3390/medicina56040174
Erskine, L., François, U., Denti, L., Joyce, A., Tillo, M., Bruce, F., Vargesson, N., & Ruhrberg, C. (2017). VEGF-A and neuropilin 1 (NRP1) shape axon projections in the developing CNS via dual roles in neurons and blood vessels. Development (Cambridge, England), 144(13), 2504–2516. https://doi.org/10.1242/dev.151621
Escudero, C., Celis, C., Saez, T., San Martin, S., Valenzuela, F. J., Aguayo, C., Bertoglia, P., Roberts, J. M., & Acurio, J. (2014a). Increased placental angiogenesis in late and early onset pre-eclampsia is associated with differential activation of vascular endothelial growth factor receptor 2. Placenta, 35(3), 207–215. https://doi.org/10.1016/j.placenta.2014.01.007
Escudero, C., Roberts, J. M., Myatt, L., & Feoktistov, I. (2014b). Impaired adenosine-mediated angiogenesis in preeclampsia: potential implications for fetal programming. Frontiers in pharmacology, 5, 134. https://doi.org/10.3389/fphar.2014.00134
Figueiró-Filho, E. A., Croy, B. A., Reynolds, J. N., Dang, F., Piro, D., Rätsep, M. T., Forkert, N. D., Paolozza, A., Smith, G. N., & Stroman, P. W. (2017a). Diffusion Tensor Imaging of White Matter in Children Born from Preeclamptic Gestations. AJNR. American journal of neuroradiology, 38(4), 801–806. https://doi.org/10.3174/ajnr.A5064
Figueiró-Filho, E. A., Mak, L. E., Reynolds, J. N., Stroman, P. W., Smith, G. N., Forkert, N. D., Paolozza, A., Rätsep, M. T., & Croy, B. A. (2017b). Neurological function in children born to preeclamptic and hypertensive mothers - A systematic review. Pregnancy hypertension, 10, 1–6. https://doi.org/10.1016/j.preghy.2017.07.144
Gehmeyr, J., Maghnouj, A., Tjaden, J., Vorgerd, M., Hahn, S., Matschke, V., Theis, V., & Theiss, C. (2021). Disabling VEGF-Response of Purkinje Cells by Downregulation of KDR via miRNA-204-5p. International journal of molecular sciences, 22(4), 2173. https://doi.org/10.3390/ijms22042173
Grace, T., Bulsara, M., Pennell, C., & Hands, B. (2014). Maternal hypertensive diseases negatively affect offspring motor development. Pregnancy hypertension, 4(3), 209–214. https://doi.org/10.1016/j.preghy.2014.04.003
Gumusoglu, S. B., Chilukuri, A. S. S., Santillan, D. A., Santillan, M. K., & Stevens, H. E. (2020). Neurodevelopmental Outcomes of Prenatal Preeclampsia Exposure. Trends in neurosciences, 43(4), 253–268. https://doi.org/10.1016/j.tins.2020.02.003
Haldipur, P., Dang, D., & Millen, K. J. (2018). Embryology. Handbook of clinical neurology, 154, 29–44. https://doi.org/10.1016/B978-0-444-63956-1.00002-3
Herrfurth, L., Theis, V., Matschke, V., May, C., Marcus, K., & Theiss, C. (2017). Morphological Plasticity of Emerging Purkinje Cells in Response to Exogenous VEGF. Frontiers in molecular neuroscience, 10, 2. https://doi.org/10.3389/fnmol.2017.00002
Ijomone, O. K., Osahon, I. R., Okoh, C. O. A., Akingbade, G. T., & Ijomone, O. M. (2021). Neurovascular dysfunctions in hypertensive disorders of pregnancy. Metabolic brain disease, 36(6), 1109–1117. https://doi.org/10.1007/s11011-021-00710-x
Ijomone, O. K., Shallie, P., & Naicker, T. (2018). Changes in the structure and function of the brain years after Pre-eclampsia. Ageing research reviews, 47, 49–54. https://doi.org/10.1016/j.arr.2018.06.006
Ives, C. W., Sinkey, R., Rajapreyar, I., Tita, A. T. N., & Oparil, S. (2020). Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. Journal of the American College of Cardiology, 76(14), 1690–1702. https://doi.org/10.1016/j.jacc.2020.08.014
Kay, V. R., Cahill, L. S., Hanif, A., Sled, J. G., Carmeliet, P., Tayade, C., & Croy, B. A. (2019a). Adult Pgf-/- mice behaviour and neuroanatomy are altered by neonatal treatment with recombinant placental growth factor. Scientific reports, 9(1), 9285. https://doi.org/10.1038/s41598-019-45824-6
Kay, V. R., Rätsep, M. T., Cahill, L. S., Hickman, A. F., Zavan, B., Newport, M. E., Ellegood, J., Laliberte, C. L., Reynolds, J. N., Carmeliet, P., Tayade, C., Sled, J. G., & Croy, B. A. (2018). Effects of placental growth factor deficiency on behavior, neuroanatomy, and cerebrovasculature of mice. Physiological genomics, 50(10), 862–875. https://doi.org/10.1152/physiolgenomics.00076.2018
Kay, V. R., Rätsep, M. T., Figueiró-Filho, E. A., & Croy, B. A. (2019b). Preeclampsia may influence offspring neuroanatomy and cognitive function: a role for placental growth factor†. Biology of reproduction, 101(2), 271–283. https://doi.org/10.1093/biolre/ioz095
Kay, V. R., Wedel, N., & Smith, G. N. (2021). Family History of Hypertension, Cardiovascular Disease, or Diabetes and Risk of Developing Preeclampsia: A Systematic Review. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC, 43(2), 227–236.e19. https://doi.org/10.1016/j.jogc.2020.08.010
Koch, S., & Claesson-Welsh, L. (2012). Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harbor perspectives in medicine, 2(7), a006502. https://doi.org/10.1101/cshperspect.a006502
Krause, D. N., Duckles, S. P., & Gonzales, R. J. (2011). Local oestrogenic/androgenic balance in the cerebral vasculature. Acta physiologica (Oxford, England), 203(1), 181–186. https://doi.org/10.1111/j.1748-1716.2011.02323.x
Lara, E., Acurio, J., Leon, J., Penny, J., Torres-Vergara, P., & Escudero, C. (2018). Are the Cognitive Alterations Present in Children Born From Preeclamptic Pregnancies the Result of Impaired Angiogenesis? Focus on the Potential Role of the VEGF Family. Frontiers in physiology, 9, 1591. https://doi.org/10.3389/fphys.2018.01591
Liu, X., Zhao, W., Liu, H., Kang, Y., Ye, C., Gu, W., Hu, R., & Li, X. (2016). Developmental and Functional Brain Impairment in Offspring from Preeclampsia-Like Rats. Molecular neurobiology, 53(2), 1009–1019. https://doi.org/10.1007/s12035-014-9060-7
Liu, Y., Ren, M., Bi, X., Fu, Y., Jing, X., Zhang, H., Cao, B., & Wang, C. (2021). A systematic review on the application of vascular endothelial growth factors in preeclampsia. Annals of palliative medicine, 10(8), 9259–9266. https://doi.org/10.21037/apm-21-2109
Luna, R. L., Kay, V. R., Rätsep, M. T., Khalaj, K., Bidarimath, M., Peterson, N., Carmeliet, P., Jin, A., & Croy, B. A. (2016). Placental growth factor deficiency is associated with impaired cerebral vascular development in mice. Molecular human reproduction, 22(2), 130–142. https://doi.org/10.1093/molehr/gav069
Magro-Malosso, E. R., Saccone, G., Di Tommaso, M., Roman, A., & Berghella, V. (2017). Exercise during pregnancy and risk of gestational hypertensive disorders: a systematic review and meta-analysis. Acta obstetricia et gynecologica Scandinavica, 96(8), 921–931. https://doi.org/10.1111/aogs.13151
Maher, G. M., O'Keeffe, G. W., O'Keeffe, L. M., Matvienko-Sikar, K., Dalman, C., Kearney, P. M., McCarthy, F. P., & Khashan, A. S. (2020). The Association Between Preeclampsia and Childhood Development and Behavioural Outcomes. Maternal and child health journal, 24(6), 727–738. https://doi.org/10.1007/s10995-020-02921-7
Mak, L. E., Croy, B. A., Kay, V., Reynolds, J. N., Rätsep, M. T., Forkert, N. D., Smith, G. N., Paolozza, A., Stroman, P. W., & Figueiró-Filho, E. A. (2018). Resting-state functional connectivity in children born from gestations complicated by preeclampsia: A pilot study cohort. Pregnancy hypertension, 12, 23–28. https://doi.org/10.1016/j.preghy.2018.02.004
Mann, J. R., McDermott, S., Griffith, M. I., Hardin, J., & Gregg, A. (2011). Uncovering the complex relationship between pre-eclampsia, preterm birth and cerebral palsy. Paediatric and perinatal epidemiology, 25(2), 100–110. https://doi.org/10.1111/j.1365-3016.2010.01157.x
Marins, L. R., Anizelli, L. B., Romanowski, M. D., & Sarquis, A. L. (2019). How does preeclampsia affect neonates? Highlights in the disease's immunity. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians, 32(7), 1205–1212. https://doi.org/10.1080/14767058.2017.1401996
Matsumoto, T., Bohman, S., Dixelius, J., Berge, T., Dimberg, A., Magnusson, P., Wang, L., Wikner, C., Qi, J. H., Wernstedt, C., Wu, J., Bruheim, S., Mugishima, H., Mukhopadhyay, D., Spurkland, A., & Claesson-Welsh, L. (2005). VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. The EMBO journal, 24(13), 2342–2353. https://doi.org/10.1038/sj.emboj.7600709
Mitoma, H., Manto, M., & Hampe, C. S. (2019). Immune-mediated Cerebellar Ataxias: Practical Guidelines and Therapeutic Challenges. Current neuropharmacology, 17(1), 33–58. https://doi.org/10.2174/1570159X16666180917105033
Mor, O., Stavsky, M., Yitshak-Sade, M., Mastrolia, S. A., Beer-Weisel, R., Rafaeli-Yehudai, T., Besser, L., Hamou, B., Mazor, M., & Erez, O. (2016). Early onset preeclampsia and cerebral palsy: a double hit model?. American journal of obstetrics and gynecology, 214(1), 105.e1–105.e1059. https://doi.org/10.1016/j.ajog.2015.08.020
Muralimanoharan, S., Maloyan, A., & Myatt, L. (2013). Evidence of sexual dimorphism in the placental function with severe preeclampsia. Placenta, 34(12), 1183–1189. https://doi.org/10.1016/j.placenta.2013.09.015
Myatt L. (2022). The prediction of preeclampsia: the way forward. American journal of obstetrics and gynecology, 226(2S), S1102–S1107.e8. https://doi.org/10.1016/j.ajog.2020.10.047
Olsson, A. K., Dimberg, A., Kreuger, J., & Claesson-Welsh, L. (2006). VEGF receptor signalling - in control of vascular function. Nature reviews. Molecular cell biology, 7(5), 359–371. https://doi.org/10.1038/nrm1911
Park, M. H., Lee, J. Y., Jeong, M. S., Jang, H. S., Endo, S., Bae, J. S., & Jin, H. K. (2018). The role of Purkinje cell-derived VEGF in cerebellar astrogliosis in Niemann-Pick type C mice. BMB reports, 51(2), 79–84. https://doi.org/10.5483/bmbrep.2018.51.2.168
Ramesar, S. V., Drewes, S. E., Gathiram, P., Moodley, J., & Mackraj, I. (2012). The effect of Kraussianone-2 (Kr2), a natural pyrano-isoflavone from Eriosema kraussianum, in an L-NAME- induced pre-eclamptic rat model. Phytotherapy research : PTR, 26(9), 1375–1380. https://doi.org/10.1002/ptr.3697
Ramesar, S. V., Mackraj, I., Gathiram, P., & Moodley, J. (2011). Sildenafil citrate decreases sFlt-1 and sEng in pregnant l-NAME treated Sprague-Dawley rats. European journal of obstetrics, gynecology, and reproductive biology, 157(2), 136–140. https://doi.org/10.1016/j.ejogrb.2011.03.005
Rana, S., Lemoine, E., Granger, J. P., & Karumanchi, S. A. (2019). Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circulation research, 124(7), 1094–1112. https://doi.org/10.1161/CIRCRESAHA.118.313276
Rätsep, M. T., Hickman, A. F., & Croy, B. A. (2016a). The Elsevier trophoblast research award lecture: Impacts of placental growth factor and preeclampsia on brain development, behaviour, and cognition. Placenta, 48 Suppl 1, S40–S46. https://doi.org/10.1016/j.placenta.2016.02.001
Rätsep, M. T., Hickman, A. F., Maser, B., Pudwell, J., Smith, G. N., Brien, D., Stroman, P. W., Adams, M. A., Reynolds, J. N., Croy, B. A., & Paolozza, A. (2016b). Impact of preeclampsia on cognitive function in the offspring. Behavioural brain research, 302, 175–181. https://doi.org/10.1016/j.bbr.2016.01.030
Rätsep, M. T., Paolozza, A., Hickman, A. F., Maser, B., Kay, V. R., Mohammad, S., Pudwell, J., Smith, G. N., Brien, D., Stroman, P. W., Adams, M. A., Reynolds, J. N., Croy, B. A., & Forkert, N. D. (2016c). Brain Structural and Vascular Anatomy Is Altered in Offspring of Pre-Eclamptic Pregnancies: A Pilot Study. AJNR. American journal of neuroradiology, 37(5), 939–945. https://doi.org/10.3174/ajnr.A4640
Reynolds, S. A., Roberts, J. M., Bodnar, L. M., Haggerty, C. L., Youk, A. O., & Catov, J. M. (2012). Newborns of preeclamptic women show evidence of sex-specific disparity in fetal growth. Gender medicine, 9(6), 424–435. https://doi.org/10.1016/j.genm.2012.10.013
Robinson, R., Lähdepuro, A., Tuovinen, S., Girchenko, P., Rantalainen, V., Heinonen, K., Lahti, J., Räikkönen, K., & Lahti-Pulkkinen, M. (2021). Maternal Hypertensive Pregnancy Disorders and Mental and Behavioral Disorders in the Offspring: a Review. Current hypertension reports, 23(5), 30. https://doi.org/10.1007/s11906-021-01141-w
Sakurai, Y., Ohgimoto, K., Kataoka, Y., Yoshida, N., & Shibuya, M. (2005). Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America, 102(4), 1076–1081. https://doi.org/10.1073/pnas.0404984102
Saputra, D., Chang, J., Lee, B. J., Yoon, J. H., Kim, J., & Lee, K. (2016). Short-term manganese inhalation decreases brain dopamine transporter levels without disrupting motor skills in rats. The Journal of toxicological sciences, 41(3), 391–402. https://doi.org/10.2131/jts.41.391
Schmahmann J. D. (2019). The cerebellum and cognition. Neuroscience letters, 688, 62–75. https://doi.org/10.1016/j.neulet.2018.07.005
Scholz, J., Niibori, Y., W Frankland, P., & P Lerch, J. (2015). Rotarod training in mice is associated with changes in brain structure observable with multimodal MRI. NeuroImage, 107, 182–189. https://doi.org/10.1016/j.neuroimage.2014.12.003
Sdrulla, A. D., & Linden, D. J. (2007). Double dissociation between long-term depression and dendritic spine morphology in cerebellar Purkinje cells. Nature neuroscience, 10(5), 546–548. https://doi.org/10.1038/nn1889
Silveira, R. C., Procianoy, R. S., Koch, M. S., Benjamin, A. C., & Schlindwein, C. F. (2007). Growth and neurodevelopment outcome of very low birth weight infants delivered by preeclamptic mothers. Acta paediatrica (Oslo, Norway : 1992), 96(12), 1738–1742. https://doi.org/10.1111/j.1651-2227.2007.00552.x
Staff, A. C., Braekke, K., Harsem, N. K., Lyberg, T., & Holthe, M. R. (2005). Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. European journal of obstetrics, gynecology, and reproductive biology, 122(1), 33–39. https://doi.org/10.1016/j.ejogrb.2004.11.015
Sun, Z., Li, X., Massena, S., Kutschera, S., Padhan, N., Gualandi, L., Sundvold-Gjerstad, V., Gustafsson, K., Choy, W. W., Zang, G., Quach, M., Jansson, L., Phillipson, M., Abid, M. R., Spurkland, A., & Claesson-Welsh, L. (2012). VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. The Journal of experimental medicine, 209(7), 1363–1377. https://doi.org/10.1084/jem.20111343
Tillo, M., Erskine, L., Cariboni, A., Fantin, A., Joyce, A., Denti, L., & Ruhrberg, C. (2015). VEGF189 binds NRP1 and is sufficient for VEGF/NRP1-dependent neuronal patterning in the developing brain. Development (Cambridge, England), 142(2), 314–319. https://doi.org/10.1242/dev.115998
Tjaden, J., Eickhoff, A., Stahlke, S., Gehmeyr, J., Vorgerd, M., Theis, V., Matschke, V., & Theiss, C. (2021). Expression Pattern of T-Type Ca2+ Channels in Cerebellar Purkinje Cells after VEGF Treatment. Cells, 10(9), 2277. https://doi.org/10.3390/cells10092277
Tuovinen, S., Aalto-Viljakainen, T., Eriksson, J. G., Kajantie, E., Lahti, J., Pesonen, A. K., Heinonen, K., Lahti, M., Osmond, C., Barker, D. J., & Räikkönen, K. (2014a). Maternal hypertensive disorders during pregnancy: adaptive functioning and psychiatric and psychological problems of the older offspring. BJOG : an international journal of obstetrics and gynaecology, 121(12), 1482–1491. https://doi.org/10.1111/1471-0528.12753
Tuovinen, S., Eriksson, J. G., Kajantie, E., & Räikkönen, K. (2014b). Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review. Journal of the American Society of Hypertension : JASH, 8(11), 832–47.e1. https://doi.org/10.1016/j.jash.2014.09.005
Tuovinen, S., Räikkönen, K., Kajantie, E., Pesonen, A. K., Heinonen, K., Osmond, C., Barker, D. J., & Eriksson, J. G. (2010). Depressive symptoms in adulthood and intrauterine exposure to pre-eclampsia: the Helsinki Birth Cohort Study. BJOG : an international journal of obstetrics and gynaecology, 117(10), 1236–1242. https://doi.org/10.1111/j.1471-0528.2010.02634.x
Tuovinen, S., Räikkönen, K., Pesonen, A. K., Lahti, M., Heinonen, K., Wahlbeck, K., Kajantie, E., Osmond, C., Barker, D. J., & Eriksson, J. G. (2012). Hypertensive disorders in pregnancy and risk of severe mental disorders in the offspring in adulthood: the Helsinki Birth Cohort Study. Journal of psychiatric research, 46(3), 303–310. https://doi.org/10.1016/j.jpsychires.2011.11.015
Vakil, P., Henry, A., Craig, M. E., & Gow, M. L. (2022). A review of infant growth and psychomotor developmental outcomes after intrauterine exposure to preeclampsia. BMC pediatrics, 22(1), 513. https://doi.org/10.1186/s12887-022-03542-5
Valencia, M., Illanes, J., Santander, O., Saavedra, D., Adaros, M., Ibarra, A., Saavedra, G., & Pascual, R. (2019). Environmental enrichment restores the reduced expression of cerebellar synaptophysin and the motor coordination impairment in rats prenatally treated with betamethasone. Physiology & behavior, 209, 112590. https://doi.org/10.1016/j.physbeh.2019.112590
Valencia, M., Santander, O., Torres, E., Zamora, N., Muñoz, F., & Pascual, R. (2022). Environmental enrichment reverses cerebellar impairments caused by prenatal exposure to a synthetic glucocorticoid. AIMS neuroscience, 9(3), 320–344. https://doi.org/10.3934/Neuroscience.2022018
van Wassenaer, A. G., Westera, J., van Schie, P. E., Houtzager, B. A., Cranendonk, A., de Groot, L., Ganzevoort, W., Wolf, H., & de Vries, J. I. (2011). Outcome at 4.5 years of children born after expectant management of early-onset hypertensive disorders of pregnancy. American journal of obstetrics and gynecology, 204(6), 510.e1–510.e5109. https://doi.org/10.1016/j.ajog.2011.02.032
Wang, R., Ma, Y., Zhan, S., Zhang, G., Cao, L., Zhang, X., Shi, T., & Chen, W. (2020). B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell death & disease, 11(1), 55. https://doi.org/10.1038/s41419-020-2252-3
Watson, E.C., Grant, Z.L. & Coultas, L. Endothelial cell apoptosis in angiogenesis and vessel regression. Cell. Mol. Life Sci. 74, 4387–4403 (2017). https://doi.org/10.1007/s00018-017-2577-y
Zhang, M., Jin, H., & Liu, X. (2022). Preeclampsia is associated with an increased risk of autism spectrum disorder (ASD): A systematic review and meta-analysis. Asian journal of surgery, 45(11), 2521–2523. https://doi.org/10.1016/j.asjsur.2022.05.133
Zhao, Y., Zheng, Y., Liu, X., Luo, Q., Wu, D., Liu, X., & Zou, L. (2018). Inhibiting trophoblast PAR-1 overexpression suppresses sFlt-1-induced anti-angiogenesis and abnormal vascular remodeling: a possible therapeutic approach for preeclampsia. Molecular human reproduction, 24(3), 158–169. https://doi.org/10.1093/molehr/gax068
Zhou, W., Wang, H., Yang, Y., Guo, F., Yu, B., & Su, Z. (2022). Trophoblast Cell Subtypes and Dysfunction in the Placenta of Individuals with Preeclampsia Revealed by Single-Cell RNA Sequencing. Molecules and cells, 45(5), 317–328. https://doi.org/10.14348/molcells.2021.0211
Zhu, X., Jiang, R., Ying, X., Li, Z., & Jiang, P. (2022). The role of ferritin and iron dextran in exacerbating preeclampsia in an L-NAME-treated rat model. Annals of translational medicine, 10(16), 889. https://doi.org/10.21037/atm-22-3675
Zuo, J., & Jiang, Z. (2020). Melatonin attenuates hypertension and oxidative stress in a rat model of L-NAME-induced gestational hypertension. Vascular medicine (London, England), 25(4), 295–301. https://doi.org/10.1177/1358863X20919798
Acknowledgements
We want to thank for the support received from Escuela de Kinesiología, Pontificia Universidad Católica de Valparaíso, Chile.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Martina Valencia-Narbona, Eloísa Torres, Fernanda Muñoz and Trinidad García. The first draft of the manuscript was written by Martina Valencia-Narbona and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Authors are responsible for correctness of the statements provided in the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethics approval
All procedures were performed according to protocols similar to the standards used in the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards and were approved by “Pontificia Universidad Católica de Valparaíso” Bioethics Committee.
Approval was obtained from the ethics committee of University Pontificia Universidad Católica de Valparaíso. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Manuscript has no associated data
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In Brief
Effect of experimental preeclampsia on locomotor learning and differential cerebellar expression of the vascular endothelial growth factor and its receptors in the female and male infant progeny of preeclamptic mothers.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valencia-Narbona, M., Torres, E., Muñoz, F. et al. Structural and functional cerebellar impairment in the progeny of preeclamptic rat mothers. Neurosci Behav Physi 53, 1283–1299 (2023). https://doi.org/10.1007/s11055-023-01503-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01503-8