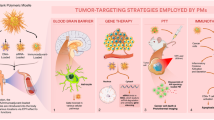
Abstract
Polymeric micelles (PMs) have gained more progress as a carrier system with the quick development of biological and nanoparticle techniques. In particular, PMs with smart targeting can deliver anti-cancer drugs directly into tumor cells at a sustained rate. PMs with core–shell structure (with diameters of 10 ~ 100 nm) have been prepared by a variety of biodegradable and biocompatible polymers via a self-assembly process. The preparation of polymeric micelles with stimuli-responsive block copolymers or modification of target molecules on polymeric micelles’ surface are able to significantly improve the efficiency of drug delivery. Polymeric micelles, which have been considered as a novel promising drug carrier for cancer therapeutics, are rapidly evolving and being introduced in an attempt to overcome several limitations of traditional chemotherapeutics, including water solubility, tumor-specific accumulation, anti-tumor efficacy, and non-specific toxicity. This review describes the preparation of polymeric micelles and the targeted modification which greatly enhance the effects of chemotherapeutic agents.








Similar content being viewed by others
References
Agut W, Brulet A, Schatz C, Taton D, Lecommandoux S (2010) pH and temperature responsive polymeric micelles and polymersomes by self-assembly of poly [2-(dimethylamino) ethyl methacrylate]-b-Poly (glutamic acid) double hydrophilic block copolymers. Langmuir 26:10546–10554
Alexis F, Rhee JW, Richie JP, Radovic-Moreno AF, Langer R, Farokhzad OC (2008) New frontiers in nanotechnology for cancer treatment. Urol Oncol 26:74–85
Bae YH (2009) Drug targeting and tumor heterogeneity. J Control Release 133:2–3
Böhmer MR, Klibanov AL, Tiemann K, Hall CS, Gruell H, Steinbach OC (2009) Ultrasound triggered image-guided drug delivery. Eur J Radiol 70:242–253
Boisseau P, Loubaton B (2011) Nanomedicine, nanotechnology in medicine. CR Phys 12:620–636
Brewer E, Coleman J, Lowman A (2011) Emerging technologies of polymeric nanoparticles in cancer drug delivery. J Nanomater. doi:10.1155/2011/408675
Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K (2011) Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol 6:815–823. doi:10.1038/nnano.2011.166
Danhier F, Feron O, Préat V (2010) To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148:135–146. http://dx.doi.org/10.1016/j.jconrel.2010.08.027
Eckman AM, Tsakalozou E, Kang NY, Ponta A, Bae Y (2012) Drug release patterns and cytotoxicity of PEG-poly (aspartate) block copolymer micelles in cancer cells. Pharm Res 29:1755–1767
Finger EC, Giaccia AJ (2010) Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metast Rev 29:285–293. doi:10.1007/s10555-010-9224-5
Fournier E, Dufresne MH, Smith DC, Ranger M, Leroux JC (2004) A novel one-step drug-loading procedure for water-soluble amphiphilic nanocarriers. Pharm Res 21:962–968
Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC (2005) Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release 109:169–188
Gong J, Chen M, Zheng Y, Wang S, Wang Y (2012) Polymeric micelles drug delivery system in oncology. J Control Release 159:312–323. http://dx.doi.org/10.1016/j.jconrel.2011.12.012
Gourevich D, Gerold B, Arditti F, Xu D, Liu D, Volovick A, Wang L, Medan Y, Gnaim J, Prentice P (2012) Ultrasound activated nano-encapsulated targeted drug delivery and tumour cell poration. Nano Biotechnol Biomed Diagn Res 733:135–144
Grande R, Carvalho AJF (2011) Compatible ternary blends of chitosan/poly (vinyl alcohol)/poly (lactic acid) produced by oil-in-water emulsion processing. Biomacromolecules 12:907–914
Gullotti E, Yeo Y (2009) Extracellularly activated nanocarriers: a new paradigm of tumor targeted drug delivery. Mol Pharm 6:1041–1051
Hammond PT (2011) Virtual issue on nanomaterials for drug delivery. ACS Nano 5:681–684. doi:10.1021/Nn2003508
He J, Zhou Z, Fan Y, Zhou X, Du H (2011) Sustained release of low molecular weight heparin from PLGA microspheres prepared by a solid-in-oil-in-water emulsion method. J Microencapsul 28:763–770
Hua SH, Li YY, Liu Y, Xiao W, Li C, Huang FW, Zhang XZ, Zhuo RX (2010) Self-assembled micelles based on PEG-polypeptide hybrid copolymers for drug delivery. Macromol Rapid Comm 31:81–86. doi:10.1002/marc.200900473
Hui G, Ma Y, Lu X, Liang Y, Chen B, Ma J (2011) pH-responsive nano-assemblies of amino poly (glycerol methacrylate). Eur Polym J 47:1232–1239
Jin Q, Liu G, Ji J (2010) Preparation of reversibly photo-cross-linked nanogels from pH-responsive block copolymers and use as nanoreactors for the synthesis of gold nanoparticles. Eur Polym J 46:2120–2128
Jones MC, Leroux JC (1999) Polymeric micelles-a new generation of colloidal drug carriers. Eur J Pharm Biopharm 48:101–111
Jungnickel C, Łuczak J, Ranke J, Fernández JF, Müller A, Thöming J (2008) Micelle formation of imidazolium ionic liquids in aqueous solution. Colloid Surf A Physicochem Eng Asp 316:278–284
Katsogiannou M, Peng L, V Catapano C, Rocchi P (2011) Active-targeted nanotherapy strategies for prostate cancer. Curr Cancer Drug Targets 11:954–965. http://dx.doi.org/10.2174/156800911797264770
Kedar U, Phutane P, Shidhaye S, Kadam V (2010) Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed Nanotechnol Biol Med 6:714–729. http://dx.doi.org/10.1016/j.nano.2010.05.005
Kim S, Lee J (2010) Effective polymeric dispersants for vacuum, convection and freeze drying of drug nanosuspensions. Int J Pharm 397:218–224
Kim GJ, Nie S (2005) Targeted cancer nanotherapy. Mater Today 8:28–33
Kim E, Jung Y, Choi H, Yang J, Suh JS, Huh YM, Kim K, Haam S (2010a) Prostate cancer cell death produced by the co-delivery of Bcl-xL shRNA and doxorubicin using an aptamer-conjugated polyplex. Biomaterials 31:4592–4599
Kim JK, Yang SY, Lee Y, Kim Y (2010b) Functional nanomaterials based on block copolymer self-assembly. Prog Polym Sci 35:1325–1349
Kim S, Shi Y, Kim JY, Park K, Cheng JX (2010c) Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin Drug Deliv 7:49–62. doi:10.1517/17425240903380446
Kwon G, Naito M, Yokoyama M, Okano T, Sakurai Y, Kataoka K (1997) Block copolymer micelles for drug delivery: loading and release of doxorubicin. J Control Release 48:195–201
Lee H, Fonge H, Hoang B, Reilly RM, Allen C (2010) The effects of particle size and molecular targeting on the intratumoral and subcellular distribution of polymeric nanoparticles. Mol Pharmaceut 7:1195–1208
Liang H, Tang J, Halliwell M (2010) Sonoporation, drug delivery, and gene therapy. P I Mech Eng H 224:343–361
Liao C, Sun Q, Liang B, Shen J, Shuai X (2010) Targeting EGFR-overexpressing tumor cells using Cetuximab-immunomicelles loaded with doxorubicin and superparamagnetic iron oxide. Eur J Radiol 80:699–705
Lin JP, Zhu JQ, Chen T, Lin SL, Cai CH, Zhang LS, Zhuang Y, Wang XS (2009) Drug releasing behavior of hybrid micelles containing polypeptide triblock copolymer. Biomaterials 30:108–117
Liu F, Park JY, Zhang Y, Conwell C, Liu Y, Bathula SR, Huang L (2010) Targeted cancer therapy with novel high drug-loading nanocrystals. J Pharm Sci 99:3542–3551
Liu Y, Sun J, Cao W, Yang J, Lian H, Li X, Sun Y, Wang Y, Wang S, He Z (2011) Dual targeting folate-conjugated hyaluronic acid polymeric micelles for paclitaxel delivery. Int J Pharm 421:160–169
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65:271–284. http://dx.doi.org/10.1016/S0168-3659(99)00248-5
Matsumura Y (2010) Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv Drug Deliv Rev 63:184–192
Matsumura Y, Kataoka K (2009) Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci 100:572–579
Mikhail AS, Allen C (2009) Block copolymer micelles for delivery of cancer therapy: transport at the whole body, tissue and cellular levels. J Control Release 138:214-223. http://dx.doi.org/10.1016/j.jconrel.2009.04.010
Minko T (2012) Receptor mediated delivery systems for cancer therapeutics. Fundam Appl Contr Release Drug Deliv 4:329–355
Mishra B, Patel BB, Tiwari S (2010) Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomed Nanotechnol Biol Med 6:9-24. http://dx.doi.org/10.1016/j.nano.2009.04.008
Miyata K, Christie RJ, Kataoka K (2010) Polymeric micelles for nano-scale drug delivery. React Funct Polym. doi:10.1016/j.reactfunctpolym.2010.10.009
Mudshinge SR, Deore AB, Patil S, Bhalgat CM (2011) Nanoparticles: emerging carriers for drug delivery. Saudi Pharm J. doi:10.1016/j.jsps.2011.04.001
Nakayama M, Okano T (2011) Multi-targeting cancer chemotherapy using temperature-responsive drug carrier systems. React Funct Polym. doi:10.1016/j.reactfunctpolym.2010.08.006
Parveen S, Misra R, Sahoo SK (2011) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed Nanotechnol Biol Med 8:147–166. http://dx.doi.org/10.1016/j.nano.2011.05.016
Pasut G, Veronese FM (2009) PEG conjugates in clinical development or use as anticancer agents: An overview. Adv Drug Deliv Rev 61:1177–1188. http://dx.doi.org/10.1016/j.addr.2009.02.010
Patravale V, Date AA, Kulkarni R (2004) Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol 56:827–840
Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. doi:10.1038/nnano.2007.387
Polyak K, Haviv I, Campbell IG (2009) Co-evolution of tumor cells and their microenvironment. Trends Genet. doi:10.1016/j.tig.2008.10.012
Ponta A, Bae Y (2010) Peg-poly (amino acid) block copolymer micelles for tunable drug release. Pharm Res 27:2330–2342
Qian F, Huang J, Hussain MA (2010) Drug-polymer solubility and miscibility: stability consideration and practical challenges in amorphous solid dispersion development. J Pharm Sci 99:2941–2947
Rapoport N (2007) Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog Polym Sci 32:962–990
Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam KH (2009) Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release 138:268–276
Ren TB, Feng Y, Zhang ZH, Li L, Li YY (2011) Shell-sheddable micelles based on star-shaped poly (ε-caprolactone)-SS-poly (ethyl glycol) copolymer for intracellular drug release. Soft Matter 7:2329–2331
Seigneuric R, Markey L, Nuyten SA, Dubernet DC, Evelo TAC, Finot E, Garrido C (2010) From nanotechnology to nanomedicine: applications to cancer research. Curr Mol Med 10:640–652
Serda RE, Godin B, Blanco E, Chiappini C, Ferrari M (2010) Multi-stage delivery nano-particle systems for therapeutic applications. BBA-Gen subjects 1810:317–329. http://dx.doi.org/10.1016/j.bbagen.2010.05.004
Simnick AJ, Amiram M, Liu W, Hanna G, Dewhirst MW, Kontos CD, Chilkoti A (2011) In vivo tumor targeting by a NGR decorated micelle of a recombinant diblock copolypeptide. J Control Release 155:144–151
Song Z, Feng R, Sun M, Guo C, Gao Y, Li L, Zhai G (2011) Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci 354:116–123
Soppimath KS, Tan DCW, Yang YY (2005) pH-triggered thermally responsive polymer core-shell nanoparticles for drug delivery. Adv Mater 17:318–323
Stohrer M, Boucher Y, Stangassinger M, Jain RK (2000) Oncotic pressure in solid tumors is elevated. Cancer Res 60:4251–4255
Strehlitz B, Reinemann C, Linkorn S, Stoltenburg R (2011) Aptamers for pharmaceuticals and their application in environmental analytics. Bioanal Rev 4:1–30. doi:10.1007/s12566-011-0026-1
Talekar M, Kendall J, Denny W, Garg S (2011) Targeting of nanoparticles in cancer: drug delivery and diagnostics. Anticancer Drug 22:949–962. doi:10.1097/CAD.0b013e32834a4554
Talelli M, Hennink WE (2011) Thermosensitive polymeric micelles for targeted drug delivery. Nanomedicine. doi:10.2217/nnm.11.91
Talelli M, Rijcken CJF, Oliveira S, der Meel R, Henegouwen PMP, Lammers T, van Nostrum CF, Storm G, Hennink WE (2011) Nanobody-shell functionalized thermosensitive core-crosslinked polymeric micelles for active drug targeting. J Control Release 151:183–192
Tanaka T, Decuzzi P, Cristofanilli M, Sakamoto JH, Tasciotti E, Robertson FM, Ferrari M (2009) Nanotechnology for breast cancer therapy. Biomed Microdevices 11:49–63
Torchilin VP (2010) Antinuclear antibodies with nucleosome-restricted specificity for targeted delivery of chemotherapeutic agents. Ther Deliv 2:257–272. doi:10.4155/TDE.10.30
Wang X, Yang L, Chen ZG, Shin DM (2008) Application of nanotechnology in cancer therapy and imaging. CA Cancer J Clin 58:97–110
Wang L, Zeng R, Li C, Qiao RZ (2009) Self-assembled polypeptide-block-poly(vinylpyrrolidone) as prospective drug-delivery systems. Colloid Surf B 74:284–292
Wang X, Wang B, Zhang Q (2011) Anti-tumor targeted drug delivery systems mediated by aminopeptidase N/CD13. Acta Pharm Sin B 1:80–83. http://dx.doi.org/10.1016/j.apsb.2011.06.002
Wei H, Cheng SX, Zhang XZ, Zhuo RX (2009) Thermo-sensitive polymeric micelles based on poly (<i>N</i>-isopropylacrylamide) as drug carriers. Prog Polym Sci 34:893–910
Xiao L, Xiong X, Sun X, Zhu Y, Yang H, Chen H, Gan L, Xu H, Yang X (2011) Role of cellular uptake in the reversal of multidrug resistance by PEG-b-PLA polymeric micelles. Biomaterials 32:5148–5157
Xiao Z, Frieder J, Teply BA, Farokhzad OC (2012) Aptamer conjugates: emerging delivery platforms for targeted cancer therapy. Drug Deliv Oncol 1263–1281. doi:10.1002/9783527634057.ch39
Xie J, Lee S, Chen X (2010) Nanoparticle-based theranostic agents. Adv Drug Deliv Rev 62:1064–1079
Yang M, Wang P, Huang CY, Ku MS, Liu H, Gogos C (2010) Solid dispersion of acetaminophen and poly (ethylene oxide) prepared by hot-melt mixing. Int J Pharm 395:53–61
Yang K, Yang CH, Li Z (2011) Synthesis and Characterization of PVA/MMT Porous Nanocomposite Prepared by Directional Freeze-Drying Method. Adv Material Res 197:253–260
Yin H, Bae YH (2009) Physicochemical aspects of doxorubicin-loaded pH-sensitive polymeric micelle formulations from a mixture of poly (l-histidine)-b-poly (l-lactide)-b-poly (ethylene glycol). Eur J Pharm Biopharm 71:223–230
Yokoyama M (2010) Polymeric micelles as a new drug carrier system and their required considerations for clinical trials. Expert Opin Drug Deliv 7:145–158
Yokoyama M (2011) Clinical applications of polymeric micelle carrier systems in chemotherapy and image diagnosis of solid tumors. J Exp Clin Med 3:151–158. http://dx.doi.org/10.1016/j.jecm. 2011.06.002
Yokoyama M, Kwon GS, Okano T, Sakurai Y, Seto T, Kataoka K (1992) Preparation of micelle-forming polymer drug conjugates. Bioconjug Chem 3:295–301
Yoshitomi T, Hirayama A, Nagasaki Y (2011) The ROS scavenging and renal protective effects of pH-responsive nitroxide radical-containing nanoparticles. Biomaterials 32:8021–8028
You JO, Almeda D, Ye GJC, Auguste DT (2010) Bioresponsive matrices in drug delivery. J Biol Eng 4:15. doi:10.1186/1754-1611-4-15
Zaman NT, Yang YY, Ying JY (2010) Stimuli-responsive polymers for the targeted delivery of paclitaxel to hepatocytes. Nano Today 5:9–14
Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W (2010) Cyclic RGD conjugated poly (ethylene glycol)-co-poly (lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release 143:136–142
Zhang L, Zhu S, Qian L, Pei Y, Qiu Y, Jiang Y (2011) RGD-modified PEG-PAMAM-DOX Conjugates: in vitro and In vivo Studies for Glioma. Eur J Pharm Biopharm 79:232–240
Author information
Authors and Affiliations
Corresponding author
Additional information
Hui Ding and Xiaojun Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ding, H., Wang, X., Zhang, S. et al. Applications of polymeric micelles with tumor targeted in chemotherapy. J Nanopart Res 14, 1254 (2012). https://doi.org/10.1007/s11051-012-1254-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1254-1