Abstract
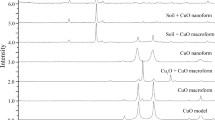
Metal oxide nanoparticles (NPs) are reported to impact plant growth in hydroponic systems. This study describes the impact of commercial CuO (<50 nm) and ZnO (<100 nm) NPs on wheat (Triticum aestivum) grown in a solid matrix, sand. The NPs contained both metallic and non-metallic impurities to different extents. Dynamic light scattering and atomic force microscopy (AFM) assessments confirmed aggregation of the NPs to submicron sizes. AFM showed transformation of ZnO NPs from initial rhomboid shapes in water to elongated rods in the aqueous phase of the sand matrix. Solubilization of metals occurred in the sand at similar rates from CuO or ZnO NPs as their bulk equivalents. Amendment of the sand with 500 mg Cu and Zn/kg sand from the NPs significantly (p = 0.05) reduced root growth, but only CuO NPs impaired shoot growth; growth reductions were less with the bulk amendments. Dissolved Cu from CuO NPs contributed to their phytotoxicity but Zn release did not account for the changes in plant growth. Bioaccumulation of Cu, mainly as CuO and Cu(I)–sulfur complexes, and Zn as Zn-phosphate was detected in the shoots of NP-challenged plants. Total Cu and Zn levels in shoot were similar whether NP or bulk materials were used. Oxidative stress in the NP-treated plants was evidenced by increased lipid peroxidation and oxidized glutathione in roots and decreased chlorophyll content in shoots; higher peroxidase and catalase activities were present in roots. These findings correlate with the NPs causing increased production of reactive oxygen species. The accumulation of Cu and Zn from NPs into edible plants has relevance to the food chain.






Similar content being viewed by others
References
Aarti PD, Tanaka R, Tanaka A (2006) Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol Plantarum 128:186–197
Akiyama M, Watanabe Y, Nishikawa T (1989) Peroxidase-activity in mast-cell granules in Urticaria pigmentosa. Dematologica 178:145–150
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31:537–548
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Asli S, Neumann PM (2009) Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ 32:577–584
Atha DH, Wang H, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing B, Nelson BC (2012) Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol 46:1819–1827
Barrena R, Casals E, Colon J, Font X, Sanchez A, Puntes V (2009) Evaluation of the ecotoxicity of model nanoparticles. Chemosph 75:850–857
Cakmak I, Horst WJ (1991) Effect of aluminum on liquid peroxidation, superoxide dismutase catalysis and peroxidase activities in root tips of soybean Glycine max. Physiol Plantarum 83:463–468
Chaoui A, El-Ferjani E (2005) Effects of cadmium and copper on antioxidant capacities, lignification and auxin degradation in leaves of pea (Pisum sativum L.) seedlings. Comptes Rendus Biol 328:23–31
Dimkpa C, Merten D, Svatoš A, Büchel G, Kothe E (2008) Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can J Microbiol 54:163–172
Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E (2009) Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol Biochem 41:154–162
Dimkpa CO, Calder C, Gajjar P, Merugu S, Huang W, Britt DW, McLean JE, Johnson WP, Anderson AJ (2011a) Interaction of silver nanoparticles with an environmentally beneficial bacterium, Pseudomonas chlororaphis. J Hazard Mater 188:428–435
Dimkpa CO, Calder A, McLean JE, Britt DW, Anderson AJ (2011b) Responses of a soil bacterium, Pseudomonas chlororaphis O6 to commercial metal oxide nanoparticles compared with responses to metal ions. Environ Pollut 159:1749–1756
Dimkpa CO, McLean JE, Britt DW, Anderson AJ (2012a) CuO and ZnO nanoparticles differently affect the secretion of fluorescent siderophores in the beneficial root colonizer, Pseudomonas chlororaphis O6. Nanotoxicology 6:635–642
Dimkpa CO, Zeng J, McLean JE, Britt DW, Zhan J, Anderson AJ (2012b) Production of indole-3-acetic acid via the indole-3-acetamide pathway in the plant-beneficial bacterium, Pseudomonas chlororaphis O6 is inhibited by ZnO nanoparticles but enhanced by CuO nanoparticles. Appl Environ Microbiol 78:1404–1410
Dimkpa CO, McLean JE, Britt DW, Johnson WP, Arey B, Lea SA, Anderson AJ (2012c) Nano-specific inhibition of pyoverdine siderophore production in Pseudomonas chlororaphis O6 by CuO nanoparticles. Chem Res Toxicol 25:1066–1074
Du W, Sun Y, Ji R, Zhu J, Wu J, Guo H (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 13:822–828
Gajewska E, Sklodowska M (2010) Differential effect of equal copper, cadmium and nickel concentration on biochemical reactions in wheat seedlings. Ecotoxicol Environ Safety 73:996–1003
Gajjar P, Pettee B, Britt DW, Huang W, Johnson WP, Anderson AJ (2009) Antimicrobial activities of commercial nanoparticles against an environmental soil microbe, Pseudomonas putida KT2440. J Biol Eng 3:9
Gao FQ, Hong FH, Liu C, Zheng L, Su MY, Wu X, Yang F, Wu C, Yang P (2006) Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach—inducing complex of rubisco–rubisco activase. Biol Trace Element Res 111:239–253
Gao F, Liu C, Qu C, Zheng L, Yang F, Su M, Hong F (2008) Was improvement of spinach growth by nano-TiO2 treatment related to the changes of rubisco activase? Biometals 21:211–217
Ghose S (1964) The crystal structure of hydrozincite, Zn5(OH)6(CO3)2. Acta Crystallogr A 17:1051–1057
Haiming F, Lintao Y, Wenshen H, Xingfang W, Zhenyu W, Sishen X, Bingsuo Z (2004) Controlled synthesis of monodispersed CuO nanocrystals. Nanotechnol 15:37
Haslett BS, Reid RJ, Rengel Z (2001) Zinc mobility in wheat: uptake and distribution of zinc applied to leaves or roots. Ann Bot 87:379–386
Haverkamp RG, Marshall AT (2009) The mechanism of metal nanoparticle formation in plants: limits on accumulation. J Nanopart Res 11:1453–1463
Hernandez-Viezcas JA, Castillo-Michel H, Servin AD, Peralta-Videa JR, Gardea-Torresdey JL (2011) Spectroscopic verification of zinc absorption and distribution in the desert plant Prosopis juliflora–velutina (velvet mesquite) treated with ZnO nanoparticles. Chem Eng J 170:346–352
Hill RJ, Jones JB (1976) Crystal-structure of hopeite. Am Mineral 61:987–995
Jouvin D, Weiss DJ, Mason TFM, Bravin MN, Louvat P, Zhao F, Ferec F, Hinsinger P, Benedetti MF (2012) Stable isotopes of Cu and Zn in higher plants: evidence for Cu reduction at the root surface and two conceptual models for isotopic fractionation processes. Environ Sci Technol 46:2652–2660
Kau LS, Spira-Solomon DJ, Penner-Hahn JE, Hodgson KO, Solomon EI (1987) X-ray absorption edge determination of the oxidation state and coordination number of copper. Application to the type 3 site in Rhus vernicifera laccase and its reaction with oxygen. J Am Chem Soc 109:6433–6442
Kim JY, Rodriguez JA, Hanson JC, Frenkel AI, Lee PL (2003) Reduction of CuO and Cu2O with H2: H embedding and kinetic effects in the formation of suboxides. J Am Chem Soc 125:10684–10692
Kropf AJ, Katsoudas J, Chattopadhyay S, Shibata T, Lang EA, Zyryanov VN, Ravel B, McIvor K, Kemner KM, Scheckel KG, Bare SR, Terry J, Kelly SD, Bunker BA, Segre CU (2010) The new MRCAT (Sector 10) bending magnet beamline at the advanced photon source. AIP Conf Proc 1234:299–302
Lee WM, An YJ, Yoon H, Kweon HS (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem 27:1915–1921
Lee WL, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez PJJ (2010) Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem 29:669–675
Lin DH, Xing BS (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Lin DH, Xing BS (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585
Lin D, Tian X, Wu F, Xing B (2010) Fate and transport of engineered nanomaterials in the environment. J Environ Qual 39:1896–1908
Lindsay WL (1979) Chemical equilibrium in soils. Wiley, New York
Liu X, Chen G, Su C (2011) Effects of material properties on sedimentation and aggregation of titanium dioxide nanoparticles of anatase and rutile in the aqueous phase. J Colloid Interface Sci 363:84–91
López-Moreno ML, de la Rosa G, Hernández-Viezcas JA, Castillo-Michel H, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2010) Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol 44:7315–7320
Manceau A, Matynia A (2010) The nature of Cu bonding to natural organic matter. Geochim Cosmochim Acta 74:2556–2580
Márquez-García B, Córdoba F (2009) Antioxidative system and oxidative stress markers in wild populations of Erica australis L. differentially exposed to pyrite mining activities. Environ Res 109:968–974
Montes-Burgos I, Walczyk D, Patrick H, Smith J, Lynch I, Dawson K (2010) Characterization of nanoparticle size and state prior to nanotoxicological studies. J Nanopart Res 12:47–53
Nair R, Varghese SH, Nair NG, Maekawa T, Yoshida Y, Kumar DS (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicol 17:372–386
Newville M (2001) IFEFFIT: interactive XAFS analysis and FEFF fitting. J Synchrotron Radiat 8:322–324
Page V, Feller U (2005) Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Ann Bot 96:425–434
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720
Panda SK, Chaudhury I, Khan MH (2003) Heavy metals induce lipid peroxidation and affect antioxidants in wheat leaves. Biol Plantarum 46:289–294
Parsons JG, Lopez ML, Gonzalez CM, Peralta-Videa JR, Gardea-Torresdey JL (2010) Toxicity and biotransformation of uncoated and coated nickel hydroxide nanoparticles on mesquite plants. Environ Toxicol Chem 29:1146–1154
Paschke MW, Redente EF (2002) Copper toxicity thresholds for important restoration grass species of the Western United States. Environ Toxicol Chem 21:2692–2697
Paschke MW, Perry LG, Redente EF (2006) Zinc toxicity thresholds for reclamation forb species. Water Air Soil Pollut 170:317–330
Perreault F, Oukarroum A, Pirastru L, Sirois L, Matias WG, Popovic R (2010) Evaluation of copper oxide nanoparticles toxicity using chlorophyll a fluorescence imaging in Lemna gibba. J Bot 763142
Pokrovsky OS, Pokrovski GS, Shirokova LS, Gonzalez AG, Emnova EE, Feurtet-Mazel A (2012) Chemical and structural status of copper associated with oxygenic and anoxygenic phototrophs and heterotrophs: possible evolutionary consequences. Geobiol 10:130–149
Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF (2003) The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66:1499–1503
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK (2007) Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12:98–105
Preis W, Gamsjäger H (2001) Solid + solute) phase equilibria in aqueous solution. XIII. Thermodynamic properties of hydrozincite and predominance diagrams for (Zn2+ + H2O + CO2. The J Chem Thermodynamics 33:803–819
Qu J, Yuan X, Wang X, Shao P (2011) Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ Pollut 159:1783–1788
Ravel B, Newville M (2005) ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12:537–541
Saison C, Perreault F, Daigle JC, Fortin C, Claverie J, Morin M, Popovic R (2010) Effect of core-shell copper oxide nanoparticles on cell culture morphology and photosynthesis (photosystem II energy distribution) in the green alga, Chlamydomonas reinhardtii. Aquatic Toxicol 96:109–114
Sarret G, Laprade PS, Bert V, Proux O, Hazemann JL, Traverse A, Marcus MA, Manceau A (2002) Forms of zinc accumulated in the hyperaccumulator Arabidopsis halleri. Plant Physiol 130:1815–1826
Shi J, Wu B, Yuan X, YY C, Chen X, Chen Y, HU T (2008) An X-ray absorption spectroscopy investigation of speciation and biotransformation of copper in Elsholtzia splendens. Plant Soil 302:163–174
Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479
Stern EA, Heald SM (1979) X-ray filter assembly for fluorescence measurements of x-ray absorption fine-structure. Rev Sci Instrum 50:1579–1582
Tait MA, Hik SD (2003) Is dimethylsulfoxide a reliable solvent for extracting chlorophyll under field conditions? Photosynth Res 78:87–91
Topnani N, Kushwaha S, Athar T (2009) Wet synthesis of copper oxide nanopowder. Int J Green Nanotechnol 1:M67–M73
Vansteveninck RFM, Babare A, Fernando DR, Vansteveninck ME (1994) The binding of zinc, but not cadmium, by phytic acid in roots of crop plants. Plant Soil 167:157–164
Voegelin A, Pfister S, Scheinost AC, Marcus MA, Kretzschmar R (2005) Changes in zinc speciation in field soil after contamination with zinc oxide. Environ Sci Technol 39:6616–6623
Warne MS, Heemsbergen D, Stevens D, McLaughlin M, Cozens G, Whatmuff M, Broos K, Barry G, Bell M, Nash D, Pritchard D, Penney N (2008) Modeling the toxicity of copper and zinc salts to wheat in 14 soils. Environ Toxicol Chem 27:786–792
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182:49–84
Yang L, Watts DJ (2005) Particle surface characteristics may play an important role in the phytotoxicity of alumina nanoparticles. Toxicol Lett 158:122–132
Zaets I, Kramarev S, Kozyrovskam N (2010) Inoculation with a bacterial consortium alleviates the effect of cadmium overdose in soybean plants. Cent Eur J Biol 5:481–490
Zhang ZY, He X, Zhang HF, Zhang P, Ding YY, Zhao YL (2011) Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 3:816–822
Zhu H, Han J, Xiao JQ, Jin Y (2008) Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit 10:713–717
Acknowledgments
This work was supported by the United States Department of Agriculture (USDA-CSREES) Grant 2009-35603-05037, the Utah Agricultural Experiment Station (Journal Paper # 8261), and the Utah Water Research Laboratory. Thanks to Moon-Juin Ngooi and Jordan Goodman for help with plant growth. For XANES data acquisition, we would like to thank John Katsoudas and Edward Lang for support at the MRCAT/EnviroCAT Sector 10BM beamline. Ken Kemner and Bhoopesh Mishra are thanked for their helpful input regarding the XAS and for help at the beamline. MRCAT operations are supported by U.S. Department of Energy (DOE) and the MRCAT member institutions. Use of the Advanced Photon Source, an Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory, is supported by the DOE under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dimkpa, C.O., McLean, J.E., Latta, D.E. et al. CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res 14, 1125 (2012). https://doi.org/10.1007/s11051-012-1125-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1125-9