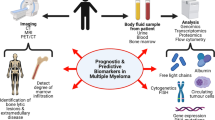
Abstract
Multiple myeloma, a complex hematologic malignancy, has devastating consequences for patients, including dramatic bone loss, severe bone pain, and pathological fractures that markedly decrease the quality of life and impact the survival of affected patients. This necessitates a refined understanding of biomarkers for accurate diagnosis and prognosis of such severe malignancy. Therefore, this article comprehensively covers current research, elucidating the diverse spectrum of biomarkers employed in clinical settings. From traditional serum markers to advanced molecular profiling techniques, the review provides a thorough examination of their utility and limitations. Through this scoping review, emphasis is placed on the evolving landscape of personalized medicine, where biomarkers play a pivotal role in tailoring therapeutic strategies. The integration of genomic, proteomic, next generation sequencing and flow cytometric data further enriches the discussion, unravelling the molecular intricacies underlying disease progression. The updated criteria allow for the treatment of people who clearly would benefit from therapy and might live longer if treated before significant organ damage occurs. Navigating through the evolving diagnostic and prognostic paradigms in multiple myeloma, this article equips clinicians and researchers with crucial insights for optimizing patient care and advancing future therapeutic approaches.



Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Rajkumar SV (2011) Treatment of multiple myeloma. Nat Reviews Clin Oncol 8(8):479–491. https://doi.org/10.1038/nrclinonc.2011.63
Rajkumar SV (2018) Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol 93(8):1091–1110. https://doi.org/10.1002/ajh.25117
Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA et al (2022) Diagnosis and management of multiple myeloma: a review. JAMA 327(5):464–477. https://doi.org/10.1001/jama.2022.0003
Grant SJ, Wildes TM, Rosko AE, Silberstein J, Giri S (2023) A real-world data analysis of predictors of early mortality after a diagnosis of multiple myeloma. Cancer 129(13):2023–2034. https://doi.org/10.1002/cncr.34760
Nieto MJ, Hedjar A, Locke M, Caro J, Saif MW (2023) Analysis of updates in multiple myeloma treatment and management. J Clin Haematol 4(1):35. https://doi.org/10.33696/haematology.4.055
Bangolo AI, Fwelo P, Trivedi C, Sagireddy S, Aljanaahi H, Auda A et al (2023) Interaction between age and gender on survival outcomes in extramedullary multiple myeloma over the past two decades. World J Clin Oncol 14(4):179. https://doi.org/10.5306/wjco.v14.i4.179
Ho M, Patel A, Goh CY, Moscvin M, Zhang L, Bianchi G (2020) Changing paradigms in diagnosis and treatment of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Leukemia 34(12):3111–3125. https://doi.org/10.1038/s41375-020-01051-x
Kyle RA, Rajkumar SV (2013) Monoclonal gammopathy of undetermined significance. Neoplast Dis Blood. https://doi.org/10.1111/j.1365-2141.2006.06235.x. 751 – 85
Van de Donk N, Mutis T, Poddighe P, Lokhorst H, Zweegman S (2016) Diagnosis, risk stratification and management of monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Int J Lab Hematol 38:110–122. https://doi.org/10.1111/ijlh.12504
Clay-Gilmour AI, Kumar S, Rajkumar SV, Rishi A, Kyle RA, Katzmann JA et al (2019) Risk of MGUS in relatives of multiple myeloma cases by clinical and tumor characteristics. Leukemia 33(2):499–507. https://doi.org/10.1038/s41375-018-0246-2
Mateos M-V, Landgren O (2016) MGUS and smoldering multiple myeloma: diagnosis and epidemiology. Plasma Cell Dyscrasias 3–12. https://doi.org/10.1007/978-3-319-40320-5_1
Brigle K, Rogers B (eds) (2017) Pathobiology and diagnosis of multiple myeloma. Seminars in oncology nursing. Elsevier. https://doi.org/10.1016/j.soncn.2017.05.012
Moore AR, Avery PR (2019) Protein characterization using electrophoresis and immunofixation; a case-based review of dogs and cats. Vet Clin Pathol 48:29–44. https://doi.org/10.1111/vcp.12760
Misra A, Mishra J, Chandramohan J, Sharma A, Raina V, Kumar R et al (2016) Old but still relevant: high resolution electrophoresis and immunofixation in multiple myeloma. Indian J Hematol Blood Transfus 32:10–17. https://doi.org/10.1007/s12288-015-0605-3
Shragai T, Gatt ME, Shaulov A, Katodritou E, Triantafyllou T, Lavi N et al (2021) Characteristics and outcome of multiple myeloma patients presenting with anaemia only: a retrospective multi-centre study. Leuk Res 101:106498. https://doi.org/10.1016/j.leukres.2020.106498
Karim KJ, Hassan AM, Getta HA, Khoshnaw NS, Jalal SD, Mohammed AM et al (2020) Frequency and prognostic significance of hypercalcemia in patients with multiple myeloma. Med J Babylon 17(4):327. https://doi.org/10.4103/MJBL.MJBL_54_20
Szabo AG, Klausen TW, Abildgaard N, Gregersen H, Silkjær T, Pedersen PT et al (2021) Incidence and clinical characteristics of multiple myeloma with low M-protein levels and normal values of hemoglobin, creatinine, calcium, and serum free light chain ratio. Blood cancer J 11(4):70. https://doi.org/10.1038/s41408-021-00460-0
Shieh AC, Faraji N, Smith D, Parikh K, Ramaiya NH (2021) Utility and clinical implications of appendicular skeleton magnetic resonance imaging in multiple myeloma: a single-institutional 15-year experience. J Comput Assist Tomogr 45(6):904–911. https://doi.org/10.1097/RCT.0000000000001195
Talamo G, Farooq U, Zangari M, Liao J, Dolloff NG, Loughran TP Jr et al (2010) Beyond the CRAB symptoms: a study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk 10(6):464–468. https://doi.org/10.3816/CLML.2010.n.080
Ramsenthaler C, Kane P, Gao W, Siegert RJ, Edmonds PM, Schey SA et al (2016) Prevalence of symptoms in patients with multiple myeloma: a systematic review and meta-analysis. Eur J Haematol 97(5):416–429. https://doi.org/10.1111/ejh.12790
Heider M, Nickel K, Högner M, Bassermann F (2021) Multiple myeloma: molecular pathogenesis and disease evolution. Oncol Res Treat 44(12):672–681. https://doi.org/10.1159/000520312
Rustad EH, Yellapantula V, Leongamornlert D, Bolli N, Ledergor G, Nadeu F et al (2020) Timing the initiation of multiple myeloma. Nat Commun 11(1):1917. https://doi.org/10.1038/s41467-020-15740-9
Abdallah N, Rajkumar SV, Greipp P, Kapoor P, Gertz MA, Dispenzieri A et al (2020) Cytogenetic abnormalities in multiple myeloma: association with disease characteristics and treatment response. Blood cancer J 10(8):82. https://doi.org/10.1038/s41408-020-00348-5
Zhu S, Li W, Lin M, Li T (2021) Serum protein electrophoresis and immunofixation electrophoresis detection in multiple myeloma. J Coll Physicians Surg Pak 30(7):864–867. https://doi.org/10.29271/jcpsp.2021.07.864
Richard S, Jagannath S, Boccadoro M, Mina R (2020) Should high-risk smouldering multiple myeloma be treated? Lancet Haematol 7(1):e15–e6. https://doi.org/10.1080/10428194.2021.1951723
Durie BG, Salmon SE (1975) A clinical staging system for multiple myeloma correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 36(3):842–854
Group IMW (2003) Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 121(5):749–757
Greipp PR, Miguel JS, Durie BG, Crowley JJ, Barlogie B, Bladé J et al (2005) International staging system for multiple myeloma. J Clin Oncol 23(15):3412–3420. https://doi.org/10.1200/JCO.2005.04.242
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L et al (2015) Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol 33(26):2863. https://doi.org/10.1200/JCO.2015.61.2267
Branagan A, Lei M, Lou U, Raje N (2020) Current treatment strategies for multiple myeloma. JCO Oncol Pract 16(1):5–14. https://doi.org/10.1200/JOP.19.00244
Alanazi F, Kwa FA, Burchall G, Jackson DE (2020) New generation drugs for treatment of multiple myeloma. Drug Discovery Today 25(2):367–379. https://doi.org/10.1016/j.drudis.2019.11.008
Das S, Juliana N, Yazit NAA, Azmani S, Abu IF (2022) Multiple myeloma: challenges encountered and future options for better treatment. Int J Mol Sci 23(3):1649. https://doi.org/10.3390/ijms23031649
Cagney DN, Sul J, Huang RY, Ligon KL, Wen PY, Alexander BM (2018) The FDA NIH Biomarkers, EndpointS, and other tools (BEST) resource in neuro-oncology. Neurooncology 20(9):1162–1172. https://doi.org/10.1093/neuonc/nox242
Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O et al (2016) Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia 30(5):1005–1017. https://doi.org/10.1038/leu.2015.356
Glavey SV, Leung N (2016) Monoclonal gammopathy: the good, the bad and the ugly. Blood Rev 30(3):223–231. https://doi.org/10.1016/j.blre.2015.12.001
Murata K, McCash SI, Carroll B, Lesokhin AM, Hassoun H, Lendvai N et al (2018) Treatment of multiple myeloma with monoclonal antibodies and the dilemma of false positive M-spikes in peripheral blood. Clin Biochem 51:66–71. https://doi.org/10.1016/j.clinbiochem.2016.09.015
Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM (2009) A monoclonal gammopathy precedes multiple myeloma in most patients. Blood J Am Soc Hematol 113(22):5418–5422. https://doi.org/10.1182/blood-2008-12-195008
Morrison T, Booth RA, Hauff K, Berardi P, Visram A (2019) Laboratory assessment of multiple myeloma. Adv Clin Chem 89:1–58. https://doi.org/10.1016/bs.acc.2018.12.001
Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT et al (2001) Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 47(4):673–680
Charafeddine KM, Jabbour MN, Kadi RH, Daher RT (2012) Extended use of serum free light chain as a biomarker in lymphoproliferative disorders: a comprehensive review. Am J Clin Pathol 137(6):890–897. https://doi.org/10.1309/AJCP4INKZ6LYAQXW
Scarpa R, Pulvirenti F, Pecoraro A, Vultaggio A, Marasco C, Ria R et al (2020) Serum free light chains in common variable immunodeficiency disorders: role in differential diagnosis and association with clinical phenotype. Front Immunol 11:319. https://doi.org/10.3389/fimmu.2020.00319
Lee WS, Singh G (2019) Serum free light chain assay in monoclonal gammopathic manifestations. Lab Med 50(4):381–389. https://doi.org/10.1093/labmed/lmz007
Barlogie B, Shaughnessy J, Munshi N, Epstein J (2001) Plasma cell myeloma. Williams Hematol 7:1501–1533
Scudla V, Budikova M, Fischerova E, Zadrazil J, Indrak K (1991) Stratification of multiple myeloma according to serum beta 2-microglobulin and serum albumin levels. Acta Universitatis Palackianae Olomucensis Facultatis Medicae 130:201–212
Dorina P, Oltean G, Smaranda D, Marcela C, Macarie I (2011) Beta-2 microglobulin as prognostic marker in multiple myeloma. Age 63:38–88
Gupta N, Sharma A, Sharma A (2020) Emerging biomarkers in multiple myeloma: a review. Clin Chim Acta 503:45–53. https://doi.org/10.1016/j.cca.2019.12.026
Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B et al (2011) Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. J Am Soc Hematol 117(18):4696–4700. https://doi.org/10.1182/blood-2010-10-300970
Kumar SK, Harrison SJ, Cavo M, de la Rubia J, Popat R, Gasparetto C et al (2020) Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 21(12):1630–1642. https://doi.org/10.1016/S1470-2045(20)30525-8
Merz M, Hielscher T, Seckinger A, Hose D, Mai EK, Raab MS, Goldschmidt H, Jauch A, Hillengass J (2016) Baseline characteristics, chromosomal alterations, and treatment affecting prognosis of deletion 17p in newly diagnosed myeloma. Am J Hematol 91(11):E473–E477. https://doi.org/10.1002/ajh.24533
Boyd KD, Ross FM, Walker BA, Wardell CP, Tapper WJ, Chiecchio L et al (2011) Mapping of chromosome 1p deletions in myeloma identifies FAM46C at 1p12 and CDKN2C at 1p32. 3 as being genes in regions associated with adverse survival. Clin Cancer Res 17(24):7776–7784. https://doi.org/10.1158/1078-0432.CCR-11-1791
Weh HJ, Gutensohn K, Selbach J, Kruse R, Wacker-Backhaus G, Seeger D et al (1993) Karyotype in multiple myeloma and plasma cell leukaemia. Eur J Cancer 29(9):1269–1273. https://doi.org/10.1016/0959-8049(93)90071-m
Barilà G, Bonaldi L, Grassi A, Martines A, Liço A, Macrì N et al (2020) Identification of the true hyperdiploid multiple myeloma subset by combining conventional karyotyping and FISH analysis. Blood cancer J 10(2):18. https://doi.org/10.1038/s41408-020-0285-6
Schmidt TM, Barwick BG, Joseph N, Heffner LT, Hofmeister CC, Bernal L, Dhodapkar MV, Gupta VA, Jaye DL, Wu J, Goyal S (2019) Gain of chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer J 9(12):94. https://doi.org/10.1038/s41408-019-0254-0
Kumar S, Fonseca R, Ketterling RP, Dispenzieri A, Lacy MQ, Gertz MA et al (2012) Trisomies in multiple myeloma: impact on survival in patients with high-risk cytogenetics. Blood J Am Soc Hematol 119(9):2100–2105. https://doi.org/10.1182/blood-2011-11-390658
Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E et al (2002) Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood J Am Soc Hematol 99(5):1745–1757. https://doi.org/10.1182/blood.v99.5.1745
Shaughnessy JD Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I et al (2007) A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood 109(6):2276–2284. https://doi.org/10.1182/blood-2006-07-038430
Decaux O, Lodé L, Magrangeas F, Charbonnel C, Gouraud W, Jézéquel P et al (2008) Prediction of survival in multiple myeloma based on gene expression profiles reveals cell cycle and chromosomal instability signatures in high-risk patients and hyperdiploid signatures in low-risk patients: a study of the Intergroupe Francophone Du Myelome. J Clin Oncol 26(29):4798–4805. https://doi.org/10.1200/JCO.2007.13.8545
Ria R, Todoerti K, Berardi S, Coluccia AML, De Luisi A, Mattioli M et al (2009) Gene expression profiling of bone marrow endothelial cells in patients with multiple myeloma. Clin Cancer Res 15(17):5369–5378. https://doi.org/10.1158/1078-0432.CCR-09-0040
van Beers EH, van Vliet MH, Kuiper R, de Best L, Anderson KC, Chari A et al (2017) Prognostic validation of SKY92 and its combination with ISS in an independent cohort of patients with multiple myeloma. Clin Lymphoma Myeloma Leuk 17(9):555–562. https://doi.org/10.1016/j.clml.2017.06.020
Zamagni E, Cavo M (2012) The role of imaging techniques in the management of multiple myeloma. Br J Haematol 159(5):499–513. https://doi.org/10.1111/bjh.12007
Xu L, Wu S (2023) New diagnostic strategy for multiple myeloma: a review. Medicine 102(52):e36660. https://doi.org/10.1097/MD.0000000000036660
Bartel TB, Haessler J, Brown TL, Shaughnessy JD Jr, van Rhee F, Anaissie E et al (2009) F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood J Am Soc Hematol 114(10):2068–2076. https://doi.org/10.1182/blood-2009-03-213280
Lecouvet FE, Boyadzhiev D, Collette L, Berckmans M, Michoux N, Triqueneaux P et al (2020) MRI versus 18 F-FDG-PET/CT for detecting bone marrow involvement in multiple myeloma: diagnostic performance and clinical relevance. Eur Radiol 30:1927–1937. https://doi.org/10.1007/s00330-019-06469-1
Dimopoulos MA, Hillengass J, Usmani S, Zamagni E, Lentzsch S, Davies FE et al (2015) Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol 33(6):657–664. https://doi.org/10.1200/JCO.2014.57.9961
Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S et al (2017) Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol 18(4):e206–e17. https://doi.org/10.1016/S1470-2045(17)30189-4
Gozzetti A, Raspadori D, Pacelli P, Antonioli E, Bestoso E, Ciofini S et al (2020) Minimal residual disease (MRD) at 10 – 6 measured by Next Generation Flow (NGF) during daratumumab consolidation therapy: analysis at 18 months follow up of DART4MM Study (Daratumumab Treatment for multiple myeloma eradication). Blood 136:7–8. https://doi.org/10.3390/jpm10030120
Oliva S, Genuardi E, Petrucci MT, D’Agostino M, Auclair D, Spadano A et al (2020) Impact of minimal residual disease (MRD) by multiparameter flow cytometry (MFC) and next-generation sequencing (NGS) on outcome: results of newly diagnosed transplant-eligible multiple myeloma (MM) patients enrolled in the forte trial. Blood 136:44–45
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P et al (2016) International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17(8):e328–e46. https://doi.org/10.1016/S1470-2045(16)30206-6
Kvorning SL, Nielsen MC, Andersen NF, Hokland M, Andersen MN, Møller HJ (2020) Circulating extracellular vesicle-associated CD163 and CD206 in multiple myeloma. Eur J Haematol 104(5):409–419. https://doi.org/10.1111/ejh.13371
Zhang L, Lei Q, Wang H, Xu C, Liu T, Kong F et al (2019) Tumor-derived extracellular vesicles inhibit osteogenesis and exacerbate myeloma bone disease. Theranostics 9(1):196. https://doi.org/10.7150/thno.27550
Caivano A, Laurenzana I, De Luca L, La Rocca F, Simeon V, Trino S et al (2015) High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumor Biology 36:9739–9752. https://doi.org/10.1007/s13277-015-3741-3
Gao S-S, Wang Y-J, Zhang G-X, Zhang W-T (2020) Potential diagnostic value of circulating miRNA for multiple myeloma: a meta-analysis. J Bone Oncol 25:100327. https://doi.org/10.1016/j.jbo.2020.100327
Yang N, Chen J, Zhang H, Wang X, Yao H, Peng Y et al (2017) LncRNA OIP5-AS1 loss-induced microRNA-410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell Death Dis 8(8):e2975–e. https://doi.org/10.1038/cddis.2017.358
Wang N, Liang X, Yu W, Zhou S, Fang M (2018) Differential expression of microRNA-19b promotes proliferation of cancer stem cells by regulating the TSC1/mTOR signaling pathway in multiple myeloma. Cell Physiol Biochem 50(5):1804–1814. https://doi.org/10.1159/000494821
Kubiczkova L, Kryukov F, Slaby O, Dementyeva E, Jarkovsky J, Nekvindova J et al (2014) Circulating serum microRNAs as novel diagnostic and prognostic biomarkers for multiple myeloma and monoclonal gammopathy of undetermined significance. Haematologica 99(3):511. https://doi.org/10.3324/haematol.2013.093500
Wu P, Agnelli L, Walker BA, Todoerti K, Lionetti M, Johnson DC et al (2013) Improved risk stratification in myeloma using a micro RNA-based classifier. Br J Haematol 162(3):348–359. https://doi.org/10.1111/bjh.12394
Hao M, Zang M, Wendlandt E, Xu Y, An G, Gong D et al (2015) Low serum Mi R-19a expression as a novel poor prognostic indicator in multiple myeloma. Int J Cancer 136(8):1835–1844. https://doi.org/10.1002/ijc.29199
Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q et al (2021) Circulating tumor cells: Biology and clinical significance. Signal Transduct Target Therapy 6(1):404. https://doi.org/10.1038/s41392-021-00817-8
Ignatiadis M, Lee M, Jeffrey SS (2015) Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res 21(21):4786–4800. https://doi.org/10.1038/s41392-021-00817-8
Garcés J-J, Cedena M-T, Puig N, Burgos L, Perez JJ, Cordon L et al (2022) Circulating tumor cells for the staging of patients with newly diagnosed transplant-eligible multiple myeloma. J Clin Oncol 40(27):3151–3161. https://doi.org/10.1200/JCO.21.01365
Garcés J-J, Bretones G, Burgos L, Valdes-Mas R, Puig N, Cedena M-T et al (2020) Circulating tumor cells for comprehensive and multiregional non-invasive genetic characterization of multiple myeloma. Leukemia 34(11):3007–3018. https://doi.org/10.1038/s41375-020-0883-0
Mishima Y, Paiva B, Shi J, Park J, Manier S, Takagi S et al (2017) The mutational landscape of circulating tumor cells in multiple myeloma. Cell Rep 19(1):218–224. https://doi.org/10.1016/j.celrep.2017.03.025
Huhn S, Weinhold N, Nickel J, Pritsch M, Hielscher T, Hummel M et al (2017) Circulating tumor cells as a biomarker for response to therapy in multiple myeloma patients treated within the GMMG-MM5 trial. Bone Marrow Transplant 52(8):1194–1198. https://doi.org/10.1038/bmt.2017.91
Lohr JG, Kim S, Gould J, Knoechel B, Drier Y, Cotton MJ et al (2016) Genetic interrogation of circulating multiple myeloma cells at single-cell resolution. Sci Transl Med 8(363):363ra147–363ra147. https://doi.org/10.1126/scitranslmed.aac7037
Kis O, Kaedbey R, Chow S, Danesh A, Dowar M, Li T et al (2017) Circulating tumour DNA sequence analysis as an alternative to multiple myeloma bone marrow aspirates. Nat Commun 8(1):15086. https://doi.org/10.1038/ncomms15086
Nijhof IS, Casneuf T, Van Velzen J, van Kessel B, Axel AE, Syed K et al (2016) CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood J Am Soc Hematol 128(7):959–970. https://doi.org/10.1182/blood-2016-03-703439
Romano A, Parrinello N, Simeon V, Puglisi F, La Cava P, Bellofiore C et al (2020) High-density neutrophils in MGUS and multiple myeloma are dysfunctional and immune-suppressive due to increased STAT3 downstream signaling. Sci Rep 10(1):1983. https://doi.org/10.1038/s41598-020-58859-x
Gupta N, Khan R, Kumar R, Kumar L, Sharma A (2015) Versican and its associated molecules: potential diagnostic markers for multiple myeloma. Clin Chim Acta 442:119–124. https://doi.org/10.1016/j.cca.2015.01.012
Schumacher TN, Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science 348(6230):69–74. https://doi.org/10.1126/science.aaa4971
Siwakoti A, Khadka S, Grimshaw AA, Giri S (2024 Jul) Association between Immunoparesis and treatment outcomes of patients with newly diagnosed multiple myeloma: a systematic review and Meta-analysis. Clin Lymphoma Myeloma Leuk 19. https://doi.org/10.1016/j.jaci.2022.12.248
Sørrig R, Klausen TW, Salomo M, Vangsted AJ, Frølund UC, Andersen KT, Klostergaard A, Helleberg C, Pedersen RS, Pedersen PT, Helm-Petersen S (2017) Immunoparesis in newly diagnosed multiple myeloma patients: effects on overall survival and progression free survival in the Danish population. PLoS ONE 12(12):e0188988. https://doi.org/10.1371/journal.pone.0188988
Heaney JL, Campbell JP, Iqbal G, Cairns D, Richter A, Child JA, Gregory W, Jackson G, Kaiser M, Owen R, Davies F (2018) Characterisation of immunoparesis in newly diagnosed myeloma and its impact on progression-free and overall survival in both old and recent myeloma trials. Leukemia 32(8):1727–1738. https://doi.org/10.1038/s41375-018-0163-4
Girmenia C, Cavo M, Offidani M, Scaglione F, Corso A, Di Raimondo F, Musto P, Petrucci MT, Barosi G (2019) Management of infectious complications in multiple myeloma patients: Expert panel consensus-based recommendations. Blood Rev 34:84–94. https://doi.org/10.1016/j.blre.2019.01.001
Shah N, Chari A, Scott E, Mezzi K, Usmani SZ (2020) B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia 34(4):985–1005. https://doi.org/10.1038/s41375-020-0734-z
Gavriatopoulou M, Ntanasis-Stathopoulos I, Dimopoulos MA, Terpos E (2019) Anti-BCMA antibodies in the future management of multiple myeloma. Expert Rev Anticancer Ther 19(4):319–326. https://doi.org/10.1080/14737140.2019.1586539
Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H et al (2013) Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol 31(33):4252. https://doi.org/10.1200/JCO.2013.51.3002
Puchades-Carrasco L, Lecumberri R, Martínez-López J, Lahuerta J-J, Mateos M-V, Prósper F et al (2013) Multiple myeloma patients have a specific serum metabolomic profile that changes after achieving complete remission. Clin Cancer Res 19(17):4770–4779. https://doi.org/10.1158/1078-0432.CCR-12-2917
Fei F, Ma T, Zhou X, Zheng M, Cao B, Li J (2021) Metabolic markers for diagnosis and risk-prediction of multiple myeloma. Life Sci 265:118852. https://doi.org/10.1016/j.lfs.2020.118852
Yue L, Zeng P, Li Y, Chai Y, Wu C, Gao B (2022) Nontargeted and targeted metabolomics approaches reveal the key amino acid alterations involved in multiple myeloma. PeerJ 10:e12918. https://doi.org/10.7717/peerj.12918
Mohamed A, Collins J, Jiang H, Molendijk J, Stoll T, Torta F et al (2020) Concurrent lipidomics and proteomics on malignant plasma cells from multiple myeloma patients: probing the lipid metabolome. PLoS ONE 15(1):e0227455. https://doi.org/10.1371/journal.pone.0227455
Dunphy K, Bazou D, Henry M, Meleady P, Miettinen JJ, Heckman CA et al (2023) Proteomic and metabolomic analysis of bone marrow and plasma from patients with extramedullary multiple myeloma identifies distinct protein and metabolite signatures. Cancers 15(15):3764. https://doi.org/10.3390/cancers15153764
Chakravarti D, LaBella KA, DePinho RA (2021) Telomeres: history, health, and hallmarks of aging. Cell 184(2):306–322. https://doi.org/10.1016/j.cell.2020.12.028
Fernandes SG, Dsouza R, Pandya G, Kirtonia A, Tergaonkar V, Lee SY, Garg M, Khattar E (2020) Role of telomeres and telomeric proteins in human malignancies and their therapeutic potential. Cancers 12(7):1901. https://doi.org/10.3390/cancers12071901
Campa D, Martino A, Varkonyi J, Lesueur F, Jamroziak K, Landi S, Jurczyszyn A, Marques H, Andersen V, Jurado M, Brenner H (2015) Risk of multiple myeloma is associated with polymorphisms within telomerase genes and telomere length. Int J Cancer 136(5):E351–E358. https://doi.org/10.1002/ijc.29101
Hyatt S Examining telomere dysfunction in multiple myeloma (Doctoral dissertation, Cardiff University)
Kumar R, Khan R, Gupta N, Seth T, Sharma A, Kalaivani M, Sharma A (2018) Identifying the biomarker potential of telomerase activity and shelterin complex molecule, telomeric repeat binding factor 2 (TERF2), in multiple myeloma. Leuk Lymphoma 59(7):1677–1689. https://doi.org/10.1080/10428194.2017.1387915
Romero M, Mosquera Orgueira A, Mejía Saldarriaga M (2024) How artificial intelligence revolutionizes the world of multiple myeloma. Front Hematol 3:1331109. https://doi.org/10.3389/frhem.2024.1331109
Acknowledgements
The authors thank Dr. Pushkal Sinduvadi Ramesh, University of Pennsylvania for his helpful comments and for proofreading the manuscript.
Funding
No funding was received in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
S.K., A.M.R., and K.P.K.- conceptualization, writing the initial draft, and revision, L.M.G., and A.D. - writing and providing critical inputs, A.P. - writing, providing essential inputs and supervision, P.S.R. - writing, creating figures and revision. All authors - read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raghunathachar, S.K., Krishnamurthy, K.P., Gopalaiah, L.M. et al. Navigating the clinical landscape: Update on the diagnostic and prognostic biomarkers in multiple myeloma. Mol Biol Rep 51, 972 (2024). https://doi.org/10.1007/s11033-024-09892-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09892-w