Abstract
Background
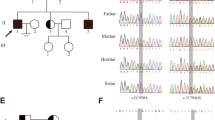
Both non-obstructive azoospermia (NOA) and primary ovarian insufficiency (POI) are pathological conditions characterized by premature and frequently complete gametogenesis failure. Considering that the conserved meiosis I steps are the same between oogenesis and spermatogenesis, inherited defects in meiosis I may result in common causes for both POI and NOA. The present research is a retrospective investigation on an Iranian family with four siblings of both genders who were affected by primary gonadal failure.
Methods
Proband, an individual with NOA, was subjected to clinical examination, hormonal assessment, and genetic consultation. After reviewing the medical history of other infertile members of the family, patients with NOA went through genetic investigations including karyotyping and assessment of Y chromosome microdeletions, followed by Whole exome sequencing (WES) on the proband. After analyzing WES data, the candidate variant was validated using Sanger sequencing and traced in the family.
Results
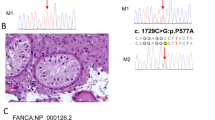
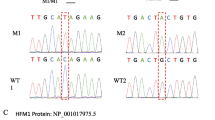
WES analysis of the proband uncovered a novel homozygote nonsense variant, namely c.118C>T in MSH4. This variant resulted in the occurrence of a premature stop codon in residue 40 of MSH4. Notably, the variant was absent in all public exome databases and in the exome data of 400 fertile Iranian individuals. Additionally, the variant was found to co-segregate with infertility in the family. It was also observed that all affected members had homozygous mutations, while their parents were heterozygous and the fertile sister had no mutant allele, corresponding to autosomal recessive inheritance. In addition, we conducted a review of variants reported so far in MSH4, as well as available clinical features related to these variants. The results show that the testicular sperm retrieval and ovarian stimulation cycles have not been successful yet.
Conclusion
Overall, the results of this study indicate that the identification of pathogenic variants in this gene will be beneficial in selecting proper therapeutic strategies. Also, the findings of this study demonstrate that clinicians should obtain the history of other family members of the opposite sex when diagnosing for POI and/or NOA.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- E2:
-
Estradiol
- FSH:
-
Follicle stimulating hormone
- LH:
-
Luteinizing hormone
- NA:
-
Not available
- NOA:
-
Non obstructive azoospermia
- NR:
-
Not reported
- POI:
-
Primary ovarian insufficiency
- PRL:
-
Prolactin
- SP:
-
No sperm retrieved after testis biopsy
- T:
-
Testosterone
- TSH:
-
Thyroid stimulating hormone
- US:
-
Ultrasonography
References
Ladjouze A, Donaldson M (2019) Primary gonadal failure. Best Pract Res Clin Endocrinol Metab 33(3):101295
POI EGGo, Webber L, Davies M, Anderson R, Bartlett J, Braat D et al (2016) ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 31(5):926–937
Hwang K, Smith JF, Coward RM, Penzias A, Bendikson K, Butts S et al (2018) Evaluation of the azoospermic male: a committee opinion. Fertil Steril 109(5):777–782
Okutman Ö (2015) Genetics of male infertility: genes implicated in non-obstructive azoospermia and severe oligozoospermia. Université de Strasbourg, Strasbourg
Kherraf Z-E, Cazin C, Bouker A, Mustapha SFB, Hennebicq S, Septier A et al (2022) Whole-exome sequencing improves the diagnosis and care of men with non-obstructive azoospermia. Am J Hum Genet 109(3):508–517
Verrilli L, Johnstone E, Allen-Brady K, Welt C (2021) Shared genetics between nonobstructive azoospermia and primary ovarian insufficiency. F&S Rev 2(3):204–213
Ward JO, Reinholdt LG, Hartford SA, Wilson LA, Munroe RJ, Schimenti KJ et al (2003) Toward the genetics of mammalian reproduction: induction and mapping of gametogenesis mutants in mice. Biol Reprod 69(5):1615–1625
Matzuk MM, Lamb DJ (2002) Genetic dissection of mammalian fertility pathways. Nat Med 8(Suppl 10):S40-S
Eskenazi S, Bachelot A, Hugon-Rodin J, Plu-Bureau G, Gompel A, Catteau-Jonard S et al (2021) Next generation sequencing should be proposed to every woman with “idiopathic” primary ovarian insufficiency. J Endocr Soc 5(7):bvab032
Chen Y, Liu X, Zhang L, Zhu F, Yan L, Tang W et al (2023) Deciphering the molecular characteristics of human idiopathic nonobstructive azoospermia from the perspective of germ cells. Adv Sci 10(17):2206852
Karakaya C, Çil AP, Bilguvar K, Çakir T, Karalok MH, Karabacak RO et al (2022) Further delineation of familial polycystic ovary syndrome (PCOS) via whole-exome sequencing: PCOS-related rare FBN3 and FN1 gene variants are identified. J Obstet Gynaecol Res 48(5):1202–1211
Fakhro KA, Elbardisi H, Arafa M, Robay A, Rodriguez-Flores JL, Mezey JG et al (2018) Point-of-care whole-exome sequencing of idiopathic male infertility. Genet Med 20(11):1365–1373
Albertsen HM, Matalliotaki C, Matalliotakis M, Zervou MI, Matalliotakis I, Spandidos DA et al (2019) Whole exome sequencing identifies hemizygous deletions in the UGT2B28 and USP17L2 genes in a three-generation family with endometriosis. Mol Med Rep 19(3):1716–1720
Wyrwoll M, Van Walree E, Hamer G, Rotte N, Motazacker M, Meijers-Heijboer H et al (2022) Bi-allelic variants in DNA mismatch repair proteins MutS Homolog MSH4 and MSH5 cause infertility in both sexes. Hum Reprod 37(1):178–189
Mukherjee S, Ridgeway A, Lamb DJ (2010) DNA mismatch repair and infertility. Curr Opin Urol 20(6):525
Kneitz B, Cohen PE, Avdievich E, Zhu L, Kane MF, Hou H et al (2000) MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev 14(9):1085–1097
Carlosama C, Elzaiat M, Patiño LC, Mateus HE, Veitia RA, Laissue P (2017) A homozygous donor splice-site mutation in the meiotic gene MSH4 causes primary ovarian insufficiency. Hum Mol Genet 26(16):3161–3166
Bache I, Assche EV, Cingoz S, Bugge M, Tümer Z, Hjorth M et al (2004) An excess of chromosome 1 breakpoints in male infertility. Eur J Hum Genet 12(12):993–1000
Barros A, Tavares M, Gomes M, Tavares M (1986) Familial inv (1)(p36. 3q12) associated with sterility. J Med Genet 23(1):90
Mandon-Pepin B, Derbois C, Matsuda F, Cotinot C, Wolgemuth D, Smith K et al (2002) Human infertility: meiotic genes as potential candidates. Gynecol obstet fertil 30(10):817–821
Stouffs K, Vandermaelen D, Tournaye H, Liebaers I, Lissens W (2011) Mutation analysis of three genes in patients with maturation arrest of spermatogenesis and couples with recurrent miscarriages. Reprod Biomed Online 22(1):65–71
Mandon-Pépin B, Touraine P, Kuttenn F, Derbois C, Rouxel A, Matsuda F et al (2008) Genetic investigation of four meiotic genes in women with premature ovarian failure. Eur J Endocrinol 158(1):107–115
Gilissen C, Hoischen A, Brunner HG, Veltman JA (2012) Disease gene identification strategies for exome sequencing. Eur J Hum Genet 20(5):490–497
Krausz C, Riera-Escamilla A, Moreno-Mendoza D, Holleman K, Cioppi F, Algaba F et al (2020) Genetic dissection of spermatogenic arrest through exome analysis: clinical implications for the management of azoospermic men. Genet Med 22(12):1956–1966
Tang D, Xu C, Geng H, Gao Y, Cheng H, Ni X et al (2020) A novel homozygous mutation in the meiotic gene MSH4 leading to male infertility due to non-obstructive azoospermia. Am J Transl Res 12(12):8185–8191
Akbari A, Padidar K, Salehi N, Mashayekhi M, Almadani N, Sadighi Gilani MA et al (2021) Rare missense variant in MSH4 associated with primary gonadal failure in both 46, XX and 46, XY individuals. Hum Reprod 36(4):1134–1145
Heddar A, Ogur C, Da Costa S, Braham I, Billaud-Rist L, Findikli N et al (2022) Genetic landscape of a large cohort of Primary Ovarian Insufficiency: New genes and pathways and implications for personalized medicine. EBioMedicine 84:104246
Luo W, Ke H, Tang S, Jiao X, Li Z, Zhao S et al (2023) Next-generation sequencing of 500 POI patients identified novel responsible monogenic and oligogenic variants. J Ovarian Res 16(1):1–13
Rahit KTH, Tarailo-Graovac M (2020) Genetic modifiers and rare Mendelian disease. Genes 11(3):239
Tukker AM, Royal CD, Bowman AB, McAllister KA (2021) The impact of environmental factors on monogenic Mendelian diseases. Toxicol Sci 181(1):3–12
Ke H, Tang S, Guo T, Hou D, Jiao X, Li S et al (2023) Landscape of pathogenic mutations in premature ovarian insufficiency. Nat Med 29(2):483–492
Li P, Ji Z, Zhi E, Zhang Y, Han S, Zhao L et al (2022) Novel bi-allelic MSH4 variants causes meiotic arrest and non-obstructive azoospermia. Reprod Biol Endocrinol 20(1):21
Acknowledgements
This study is related to the Project No. 43005818 from School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We appreciate the proband and his family for participating in this study.
Funding
This work was financially supported by Shahid Beheshti University of Medical Sciences Grant No. 43005818.
Author information
Authors and Affiliations
Contributions
Conception and design (SHS and MDO), acquisition of samples and data (SHS and EH), analysis and interpretation of data (SHS, EH and SGF), and manuscript preparation (SHS and SGF). All the authors read and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared they have no conflict of interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the National Research Committee of the Institutional Review Board (IRB), with the approval code of IR.SBMU.MSP.REC.1402.326 and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all of the participants in the study and. Written informed consent for publication of clinical details and clinical images was obtained from the all of the participants in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hashemi Sheikhshabani, S., Ghafouri-Fard, S., Hosseini, E. et al. A novel homozygote nonsense variant of MSH4 leads to primary ovarian insufficiency and non-obstructive azoospermia. Mol Biol Rep 51, 68 (2024). https://doi.org/10.1007/s11033-023-09000-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09000-4