Abstract
Background
The plant trihelix gene family is among the earliest discovered transcription factor families, and it is vital in modulating light, plant growth, and stress responses.
Methods
The identification and characterization of trihelix family members in the sesame genome were analyzed by bioinformatics methods, and the expression patterns of sesame trihelix genes were assessed by quantitative real-time PCR.
Results
There were 34 trihelix genes discovered in the genome of sesame, which were irregularly distributed among 10 linkage groups. Also, the genome contained 5 duplicate gene pairs. The 34 trihelix genes were divided into six sub-families through a phylogenetic study. A tissue-specific expression revealed that SiTH genes exhibited spatial expression patterns distinct from other trihelix genes in the same subfamily. The cis-element showed that the SiTHs gene promoter contained various elements associated with responses to hormones and multiple abiotic stresses. Additionally, the expression patterns of 8 SiTH genes in leaves under abiotic stresses demonstrated that all selected genes were significantly upregulated or downregulated at least once in the stress period. Furthermore, the SiTH4 gene was significantly induced in response to drought and salt stress, showing that SiTH genes may be engaged in the stress response mechanisms of sesame.
Conclusion
These findings establish a foundation for further investigation of the trihelix gene-mediated response to abiotic stress in sesame.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcription factors (TFs) binding to a cis-regulatory element in promoter or enhancer DNA activate or repress target gene expression, affecting plant growth and development [1, 2]. The trihelix gene family belongs to the first known TFs reported in plants [3] and was named after the presence of a specific three-helix structure (helix-loop-helix-loop-helix) in the DNA structural domain; in addition, this family is known as a GT factor because its structural domain specifically binds to GT elements necessary for the light response [4, 5]. All plant trihelix subfamilies contain one or two conserved three-helix structural domains, although their C-terminal structural domains vary [6]. The conserved domains of the trihelix family are similar to the three α-helices (helix-helix-turn-helix) of the MYB gene family, but the amino acid sequence between the two α-helices is longer; thus, their three-dimensional conformations are different, allowing them to recognize different DNA elements and bind specifically to GT elements on the DNA sequence [7]. In accordance with the characteristics of its conserved structural domains, Ruth categorized the trihelix family into five subfamilies (GT-1, GT-2, SH4, GTγ, and SIP1) [6]. Recently, Yu discovered a new subfamily, GTδ, in tomato [8].
The trihelix family exists widely in terrestrial plants [9]. Preliminary studies have concentrated on the regulation of the light response [4, 10]. The GT-1 factor, the first trihelix member found in Pisum sativum, binds to the GT element in the light-induced rbcS-3 A gene promoter [11]. Previous studies demonstrated that trihelix genes are associated with numerous biological processes, such as the regulation of late embryogenesis, and the formation of the perianth, stomata, and seeds [6, 12, 13]. The identification and cloning of trihelix genes from different subfamilies revealed their essential role in coping with stressful environments [14]. Li found that ASR3 (At2g33550) in Arabidopsis thaliana exerted a negative effect on modulating immune-related responses [15]. Three rice trihelix genes (OsGTγ-1, OsGTγ-2 and OsGTγ-3 ) and two soybean trihelix genes (GmGT-2 A and GmGT-2B) are involved in cold, drought, and salt stress responses [16, 17]. AtGT2L expression in Arabidopsis was induced during cold and salt stress [18]. Furthermore, there was evidence that trihelix genes are engaged in the abiotic stress response of maize [19].
Sesame is an ancient and traditional oilseed crop that provides a major supply of plant oil. Sesame seeds are rich in fat and protein [20]. However, the occurrence of environmental pressures in recent years, including salt, drought, and other adverse conditions, severely diminished the yield and quality of sesame [21,22,23]. With the complete sequencing of the sesame genome, many TFs involved in biotic and abiotic stresses in sesame were identified, such as NAC [24], WRKY [25], BZIP [26], HD-ZIP [27], and MATE [28]. Presently, there is not a comprehensive genomic investigation of the sesame trihelix family yet. It is necessary to identify and analyze the trihelix transcription factor family in sesame due to its potential role in stress responses.
Materials and methods
Genome-wide identification of trihelix family in sesame
The sesame genome sequence and annotation information were downloaded from the Ensembl Plants database. (http://plants.ensembl.org/index.html). The trihelix protein sequence of Arabidopsis was downloaded from TAIR (https://www.arabidopsis.org/). BLASTp alignment of Arabidopsis trihelix protein sequences to sesame protein sequences, screened for high homology, with an E-value cut-off of 1 e− 5. The Hidden Markov Model (HMM) file (PF13837) was downloaded from the PFAM database (http://pfam.xfam.org/), and the sesame genome was searched by Simple HMM Search, a built-in plug-in of TBtools [29]. The sesame trihelix protein was screened with an E-value 1 e− 5 as the threshold. BLASTp and HMM searches for candidate proteins were conducted, their protein sequences were downloaded, and whether they contained conserved domains peculiar to the trihelix family in SMART (http://smart.embl-heidelberg.de/) and NCBI-CDD (https://www.ncbi.nlm.nih.gov/cdd) was confirmed. We eliminated proteins without typical domains. The molecular weight, isoelectric point, and amino acid size of sesame trihelix protein were predicted and determined on the ExPASy website (https://www.expasy.org). WoLF PSORT (https://wolfpsort.hgc.jp/) was utilized to predict the subcellular localization of trihelix proteins.
Phylogenetic and homology analysis
ClustalW was used to accomplish multiple sequence alignment of trihelix family proteins. The best model was built with MEGAX(https://www.megasoftware.net), and the phylogenetic tree was created with the Neighbour-Joining (NJ) approach. The bootstrap parameter was set to 1000, while the other parameters were left at their default values. Use the online tool Evolview (https://evolgenius.info/evolview) to edit and enhance the phylogenetic tree image. The McScanX software in TBtools was used to examine collinearity among sesame trihelix genes, and an intraspecific collinearity diagram was drawn.
Gene structure analysis and conserved motif identification
The GSDS (http://gsds.cbi.pku.edu.cn) online produced the SiTH gene structure. To compare the differences in SiTHs, the conserved protein motifs in sesame trihelix proteins were identified and analyzed by the online search program, MEME (http://meme.nbcr.net/meme/intro.html). The relevant parameters were set as follows: The width of the motif was 6 to 50 amino acids, and the number of motifs requested was 12. Subsequently, the resulting data was portrayed in TBtools.
Analysis of cis-acting elements
TBtools software was used to extract the 2000 bp sequence upstream of the initiation codon of the trihelix gene. Plant Care (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/), an online promoter analysis tool, was used for detecting cis-acting elements implicated in abiotic stress in the SiTH genes. TBtools software was used for visual display.
Plant material growth and stress treatment
Sesame samples from different tissues and plump sesame seeds of “Zhongzhi 13”, were selected and sown in germination boxes. After being grown for 5 days in a greenhouse with a cycle of 16 h of light and 8 h of darkness, the seedlings could be transferred to a hydroponic tank and cultivated in 1/2 Hoagland nutrient solution. Roots, stems, and leaves were collected after 2 weeks of seedling growth. The tissues of flowers, capsules, and seeds were sampled during flowering. After freezing in liquid nitrogen, the samples were preserved in the refrigerator at − 80 °C for subsequent analysis. Stress treatment and sampling: The same two-week-old sesame seedlings were chosen for drought, salt, low temperature, and abscisic acid (ABA) protocols for preculture conditions. Stress treatment: Low temperature treatment transported sesame seedlings from the greenhouse to a lit incubator at 4 °C, whereas other treatments transported seedlings to 1/2 Hoagland nutrient solution with 150 mmol/L NaCl (salt stress), 15% PEG 6000 (drought stress), and 100 µM ABA. Sampling: take fresh sesame leaves at 0 h, 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h; DEPC water washed leaves; dried by filter papers; placed in a 2.0 mL centrifuge tube; frozen in liquid nitrogen in a -80 °C refrigerator. Each treatment and time point had three biological duplicates taken.
Total RNA extraction and quantitative PCR analysis
Total RNA was obtained from different tissues of sesame grown under control cultivation and leaf samples grown under abiotic stress (drought, salt, low temperature, and ABA). Total RNA used the Plant RNApure Kit (Zomanbio, Beijing) and detected by electrophoresis. The utilization of a reverse transcription kit allowed for the synthesis of cDNA (SR511, Beijing Jinsha Biotechnology Co., Ltd). The test instrument was LightCycler96 (Roche, USA), and Takara RR820A was used for qRT-PCR. Procedure extension referenced receptor instructions. Repeat genetically and biologically three times each. The SiH3.3 gene was used as an internal reference gene. The 2−ΔΔCT method served to calculate the relative gene expression levels under abiotic stress. The 2−ΔCT method served to calculate the relative gene expression levels in different tissues [30]. A t-test was used to analyze the significance of relative expression data. The Primer3 Plus online website (Primer3Plus-Pick Primers) was used to design primers. Primers were synthesized by Bioengineering (Shanghai) Co., Ltd. Table S1 contains all of the primer information.
Results
Identification of trihelix gene family in sesame
34 trihelix genes were identified in the sesame genome by homologous BLAST and HMM domain searches. Table 1 summarized the characteristics of these trihelix genes, including gene ID, protein length, gene location, protein molecular weight (MW/KD), isoelectric point (PI), and sub-cellular localization. The predicted length of sesame trihelix proteins varied from 171 aa (SiTH19) to 826 aa (SiTH12), and their molecular weight ranged from 19.95 KD (SiTH19) to 91.80 KD (SiTH12). The isoelectric point of SiTH proteins ranged from 4.61 (SiTH10) to 9.83 (SiTH28). The subcellular localization prediction of 34 trihelix proteins showed that most SiTH proteins were located in the nucleus, 3 trihelix proteins (SiTH1, SiTH2, and SiTH12) were in the cytoplasm, SiTH4 was predicted to be in the peroxisome, and SiTH20 was predicted to be in the chloroplast.
Chromosome localization and gene replication analysis
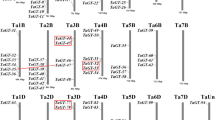
According to information on chromosome annotation, 34 trihelix genes of sesame were unequally distributed in 10 linkage groups (LG) (Fig. 1). Each linkage group contained 1 to 5 genes. Among these groups, the LG1, LG3, and LG5 linkage groups contained five trihelix genes, whereas the LG10, LG12, and LG15 linkage groups contained only one SiTH gene. No SiTH genes were detected in the LG4, LG7, LG9, LG13, LG14, and LG16 linkage groups.
To reveal the evolutionary mechanism of the trihelix gene family in sesame, duplication events were analyzed. The results demonstrated that the trihelix gene family underwent tandem and segmentary duplication. Three pairs of trihelix genes in sesame were identified as tandem duplicates (Fig. 1). Five pairs of SiTHs had segmental repeat events, as shown in Fig. 2. This result indicated that repeated gene events played an important role in amplifying the sesame trihelix gene family.
Phylogenetic analysis
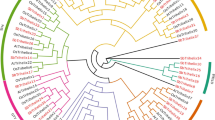
To reveal the phylogenetic relationship of the sesame trihelix family, we generated a phylogenetic tree from the amino acid sequences of trihelix proteins from sesame and other crops (Arabidopsis, tomato, soybean, and rice) by MEGAX software. The analysis found that the SiTH genes were classified into six subfamilies (Fig. 3), including SIP1, GTγ, GTδ, GT-1, GT-2, and SH4. Similar findings were also found in Arabidopsis and tomato [8]. SIP1 was the largest subfamily of sesame, containing 9 SiTH genes. The smallest group was the SH4 subfamily, which contained 3 SiTH genes. Moreover, the numbers of SiTHs in the GTγ, GTδ, GT-1 and GT-2 subfamilies were 5, 5, 7 and 5, respectively. The phylogenetic tree showed that the genetic similarity between SiTH7 and SiTH11 were highly similar (approaching 100%) in the GTγ subfamily. In the GT-2 subfamily, SiTH8 and SiTH14 exhibited strong similarities. In the SIP1 subfamily, the similarity between SiTH3 and SiTH10 genes was also approximately 100%. These gene pairs showed a closer relationship compared to others, indicating that they may perform similar functions directly.
Phylogenetic analysis of 86 trihelix proteins from Arabidopsis, rice, tomato, soybean and Sesamum indicum L. The phylogenetic tree was built with MEGAX, then edited and beautified with Evolview, an online tool. The white circle, green circle, pink circle, sky blue circle and black star represent trihelix proteins in Arabidopsis, rice, tomato, soybean and Sesamum indicum L., respectively
Gene structure and conserved motif analysis
To identify the differences among trihelix family genes in sesame, we evaluated their gene structure and conserved motifs. The number and distribution of exons and introns of 34 sesame trihelix genes were shown in Fig. 4C. The number of exons of 34 SiTH genes was discontinuously distributed from 1 to 16. More than half of the CDS of SiTH genes were separated by introns. Most of the assembled trihelix members had a similar ratio of exons to introns (Fig. 4A), and the intron trihelix transcription factor family members were all from the GTγ and SIP1 subfamilies, which was similar to the identification results in Fagopyrum tataricum and rice [9, 31]. All members of the GTγ subfamily contained one exon. SiTH5, a member of the SIP1 subfamily, contains 2 exons, and the other 8 members contained only one exon. Except for SiTH21, GT-2 subfamily members contained two exons. SH4 subfamily members contained 2 to 3 exons, and GTδ subfamily genes usually included 2 to 4 exons. SiTH23 in the GT-1 subfamily had the largest number of exons, containing 16. SiTH25 had 4 exons and other members contained 2 to 3 exons. This indicated that there are some differences in the structure of SiTH genes.
To further analyze the origin and evolutionary pattern of SiTHs, we identified 12 conserved motifs in sesame SiTH proteins using the MEME program. As shown in Fig. 4B, most SiTH proteins contained motif 1 and motif 4. SH4 subfamily members contain motifs 1, 4, and 6 (SiTH6 lacks motif 6). SiTH23 lacked motif 12, while other GT-1 subfamily members contained motifs 1, 4, 10, and 12, the SiTH1 protein contained motif 8, and SiTH32 contained motif 6. Motif 2 and motif 11 only exist in the GTδ subfamily, and most GTδ subfamily members contained motifs 1, 2, 3, and 5. The SiTH members of the GT-2 subfamily contained motifs 1, 2, 3, 5, and 9. Interestingly, all members of this subfamily had two motifs 4 and 7, which was consistent with the characteristics of the GT-2 subfamily containing two trihelix domains. The conserved motifs of all member proteins in the GTγ subfamily were motifs 1, 4, 5, and 8. This means that members of the same subfamily had similar conserved motif compositions, and there were differences between different subpopulations, which might represent different functions of the trihelix family in sesame.
The conserved motifs and gene structure of sesame trihelix family. A Phylogenetic relationships of the SiTH proteins. B Putative motifs in the 34 SiTH proteins. Conserved motifs were identified using MEME. The length of each protein could be estimated using the scale at the bottom. C The structures of 34 SiTH genes. CDS and introns are indicated by black lines and green boxes, respectively
Analysis of cis-acting elements of SiTHs
Cis-acting elements are involved in the regulation of gene expression[32, 33]. To explore the possible mechanism by which SiTH genes regulate expression, the 2000 bp sequence upstream of the start codon of trihelix gene in sesame was extracted, and the potential cis-acting elements in the promoter region were screened by Plant Care online tool. We predicted 11 types of cis-acting elements in sesame SiTHs, and element information is shown in Table S2 and Table S3. Figure 5 show that 79.4% of SiTHs contain more than 5 cis-elements. All SiTHs contained light responsive elements, which was consistent with the involvement of the trihelix gene family in the light response. Anaerobic inducible elements were more widely distributed than other abiotic stress-related elements, and 88.2% of SiTHs contained at least one anaerobic inducible element. Wound responsive elements, defense and stress responsive elements, low temperature responsive elements and drought inducible elements were detected in the promoter regions of 3, 11, 18 and 12 SiTH genes, respectively.
Additionally, hormone-responsive elements were detected, such as ABA responsive elements, gibberellin responsive elements, auxin responsive elements, MeJA responsive elements, and salicylic acid responsive elements. Among them, 24 genes contained ABA-related response elements, GA responsive elements were detected in 21 members, MeJA responsive elements were detected in 18 members, SA responsive elements were detected in 12 members, and auxin responsive elements were detected in 9 members. Moreover, some promoter elements were peculiar, such as wound response elements, which were only identified in SiTH6, SiTH17 and SiTH21. The promoter region of the trihelix gene family of sesame had a variety of cis-acting elements related to hormonal and abiotic stress, indicating that the trihelix family gene might be involved in the growth and development of sesame. Moreover, they might participate in the regulation of various abiotic stress responses.
Tissue-specific expression profiling of SiTHs
To analyze the expression of SiTHs in various tissues and organs, we measured the expression profiles of SiTH genes under normal growth conditions in six tissues (root, stem, leaf, flower, seed, and capsule) of sesame by qRT-PCR. The heatmap showed that the expression levels of SiTH genes were lower than those of the internal reference genes, but the expression patterns were different in different tissues (Fig. 6). The relative expression levels of SiTH15 in roots, stems, leaves and seeds were the highest. The expression of SiTH8 was the highest in flowers. SiTH5 was dominantly expressed in the capsule. The relative expression levels of 10 (SiTH1, SiTH3, SiTH4, SiTH9, SiTH22, SiTH23, SiTH26, SiTH32 and SiTH33) genes in 6 different tissues were relatively low. The expression patterns of SiTH genes family members in sesame tissues were not consistent, which provided a reference for further study of the role of SiTH genes in sesame growth and development.
Heatmap of relative expression levels of the SiTH genes in different tissues. The relative expression data was obtained from qRT‒PCR calculated by using SITH3.3 as the internal reference gene. The mapping data are shown in Table S4
Analysis of SiTH expression patterns under abiotic stress
To preliminarily identify the expression pattern of SiTHs under abiotic stress, 8 genes were selected for qRT-PCR detection according to the results of evolutionary tree classification and cis-acting element analysis. The results of drought treatment (Fig. 7A-H) showed that SiTH4 gene expression was upregulated at 12 h; SiTH8 was upregulated at 2 h, 4 h, 8 h, and 12 h, and the upregulation was significant at 4 and 8 h. SiTH34 was upregulated at 1 and 2 h, SiTH27 expression was significantly upregulated at 8 h, and the expression of other genes was downregulated by drought treatment. The results of salt stress treatment (Fig. 7I-P) showed that 4 genes (SiTH4, SiTH5, SiTH27 and SiTH34) were upregulated at almost time points, SiTH4 and SiTH5 genes reached upregulation peaks (approximately 4 times, 3.5 times) at 8 h, and other genes were downregulated in response to salt stress. Under cold treatment (Fig. 7Q-X), 4 genes (SiTH4, SiTH8, SiTH15, and SiTH34) were upregulated at least two time points, and SiTH4 and SiTH15 genes peaked at 1 and 12 h (approximately 7 times, 2.5 times, respectively). In summary, we found that the expression patterns of several SiTH genes under different abiotic stresses exhibit certain similarities. SiTH4 and SiTH34 were upregulated under drought, salt and cold treatment, while SiTH17 and SiTH21 were both down-regulated. However, the expression patterns of the same gene were different when participating in different stresses. SiTH8 was significantly upregulated under drought and cold stress, but it was downregulated at the next time point under salt stress, and significantly downregulated at 1 h of treatment (about 2 times). SiTH5 tended to be downregulated under drought and low temperature stress, and upregulated under salt stress. These results illustrate that these SiTH genes may play a crucial role in the response to multiple abiotic stresses in sesame.
Abscisic acid (ABA) is an important plant hormone involved in various processes of growth, development, and interaction between plants and the environment. SiTH genes are rich in ABA cis-acting elements. The 8 selected SiTH genes except SITH17 included at least one ABA response element. SITH27 contained the most ABA cis-acting elements (containing 7). Under ABA treatment, the expression of SiTH4, SiTH27, and SiTH34 was induced at least one time point (Fig. 7Y-AF). The expression of the SiTH4 gene peaked at 8 h and then decreased, but it was still at the upregulation level. The expression of the SiTH34 gene was upregulated 1 h after ABA treatment, but it was downregulated at subsequent practice time points. The expression of SiTH27 gene continued to be significantly induced with ABA treatment and peaked at 24 h. Other SiTH genes were downregulated at each processing time point.
The relative expression levels of 8 selected trihelix genes under abiotic stress as revealed by qRT‒PCR. (A–H) The relative expression levels of selected genes under drought treatment; (I–P) The relative expression levels of selected genes under salt treatment; (Q–X) The relative expression levels of selected genes under cold treatment; (Y–AF) The relative expression levels of 8 SiTH genes under ABA treatment. Statistical significance was determined using t-tests (***p < 0.001, **p < 0.01, *p < 0.05, n = 3)
Discussion
Characterization of the sesame trihelix gene
The trihelix gene family plays an important role in the regulation of plant light response [34], leaf development [35], stomatal regulation [36], seed development [13, 37, 38], and biotic and abiotic stress responses [39, 40]. In recent years, the trihelix transcription factor family has already been identified in many plants, and its functions have also been investigated [19]. In this research, bioinformatics analysis of the sesame trihelix gene family was undertaken for the first time. 34 trihelix members were identified in sesame, which were irregularly distributed among 10 linkage groups. The length of these SiTH proteins ranged from 171 to 826 aa, the isoelectric point was 4.61 to 9.83, and the molecular weight was 19.95 to 91.80 KD. 29 SiTH proteins were hypothesized in the nucleus, and the remaining 5 genes were in the peroxisome, cytoplasm, and chloroplast. These genes may have very different functions from other trihelix genes.
Gene duplication is a key process in the evolution of new genes. The development of novel gene functions in response to changing environmental conditions is facilitated by these duplications [41, 42]. In most cases, gene families are amplified primarily through the following mechanisms: tandem duplication and segmental duplication [43]. Segmental replication is the main driving force for gene family amplification. In this study, only 3 pairs of tandem genes and 5 pairs of segmental duplication genes (29.41%) were found among sesame trihelix members, this indicated that more than half of them may not be related. These results explained the differences in the structure and conserved motifs of different subfamilies of SiTH proteins to some extent. Similar phenomena also occur in other crops, such as 71 soybean trihelix genes containing only 13 pairs of segment repeat genes, and only 6 pairs of repeat genes were contained among the 41 rice trihelix genes [31, 44].
Phylogenetic analysis allows revealing gene evolution. The 34 SiTH genes were divided into 6 subfamilies, among which SIP1 was the richest subfamily, containing 9 SiTH genes. The members of the sesame trihelix family containing 1–2 introns accounted for 38.24% of the total, which was similar to the results in soybean [16]. Trihelix genes belonging to one subfamily have similar structures in sesame, such as the length and distribution of conserved motifs are consistent, which may be associated with specific functions. The difference before different subfamilies is large, which indicates that the trihelix gene is not close in evolution. Trihelix genes have been lost or expanded during the evolution of sesame, which creates a potential for the formation of new genes.
Expression analysis of sesame trihelix genes
Trihelix gene family is engaged in plant growth. In this study, we obtained the expression profiles of members of the sesame trihelix gene family in various tissues by qRT-PCR. Compared with the internal reference genes, the expression levels of SiTH genes were lower, and the expression patterns of different tissues were different. Among all members, SiTH15 exhibited the highest expression in four tissues (roots, stems, leaves, and seeds), indicating that it may take an active impact in sesame growth. SiTH5 had the highest expression in capsule and very low expression in seed. Different tissue expression patterns of SiTHs mean that members of the sesame trihelix family may have different functions.
With the frequent occurrence of extreme climate, plants are facing more severe survival conditions such as drought, salinization, and low temperature [45]. Many transcription factors are involved in regulating stress tolerance in plants. The trihelix members have been demonstrated to be crucial in the regulatory mechanisms of abiotic stress tolerance, which includes resistance to conditions like drought, salt, and low temperature. In Arabidopsis, AST1 (At3g24860) mediated salt and osmotic stress tolerance [46]; GT-2 like 1 (AtGTL1, At1g33240) inhibited SDD1 to modify stomatal density and drought tolerance; GT-4 (AT3G25990) adjusted Cor15A for salt tolerance [47]. The first Ca2+/CaM− binding trihelix transcription factor, AtGT2 (At5G28300), regulated low temperature and salinity stress [18]. ABA, a common stress hormone, may cause plant responses to various stressors [48]. Recent studies have revealed that the trihelix gene may adopt to drought and salinity stress via the ABA pathway. BnSIP1-1 mediated ABA synthesis, signal transduction, salt response, and osmotic stress. Overexpression of the BnSIP1-1 gene decreased rapeseed seedlings sensitivity to drought and ABA stress [49]. In this research, we detected that 73.68% of SiTH genes contained ABA response elements. The trihelix gene may be involved in response to abiotic stress through multiple cis-acting element regulatory pathways. Further analysis of the expression patterns of eight sesame trihelix genes in response to abiotic stress showed that the SiTH4 and SiTH34 genes were activated under drought, salt and cold treatment, whereas the SiTH17 and SiTH21 genes showed downregulated. Most selected SiTH genes expressed under ABA stress, but their expression varied substantially at different processing periods.
Conclusions
In the sesame genome, we identified 34 members of the trihelix gene family and clustered them into 6 subfamilies. The same subfamily genes usually have similar structures and conserved functional domains. Stress tolerance candidate genes were found by examining SiTH gene expression profiles under drought, salt, low temperature, and ABA stress. This study provides a basis for elucidating the function and molecular mechanism of trihelix genes in plant development and stress tolerance.
Data Availability
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
References
Franco-Zorrilla JM, López-Vidriero I, Carrasco JL, Godoy M, Vera P, Solano R (2014) DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci U S A 111(6):2367–2372. https://doi.org/10.1073/pnas.1316278111
Inukai S, Kock KH, Bulyk ML (2017) Transcription factor-DNA binding: beyond binding site motifs. Curr Opin Genet Dev 43:110–119. https://doi.org/10.1016/j.gde.2017.02.007
Zhang Q, Zhong T, Xu EL, Dai M, Sun W, Ye S J (2021) GT factor ZmGT-3b is Associated with Regulation of Photosynthesis and Defense Response to Fusarium graminearum infection in Maize Seedling. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.724133
Nagano Y, Inaba T, Furuhashi H, Sasaki Y (2001) Trihelix DNA-binding protein with specificities for two distinct cis-elements: both important for light down-regulated and dark-inducible gene expression in higher plants. J Biol Chem 276(25):22238–22243. https://doi.org/10.1074/jbc.M102474200
Zhou DX (1999) Regulatory mechanism of plant gene transcription by GT-elements and GT-factors. Trends Plant Sci 4(6):210–214. https://doi.org/10.1016/s1360-1385(99)01418-1
Kaplan-Levy RN, Brewer PB, Quon T, Smyth DR (2012) The trihelix family of transcription factors–light, stress and development. Trends Plant Sci 17(3):163–171. https://doi.org/10.1016/j.tplants.2011.12.002
Nagano Y (2000) Several features of the GT-Factor Trihelix Domain Resemble those of the myb DNA-Binding domain. Plant Physiol 124(2):491–494. https://doi.org/10.1104/pp.124.2.491
Yu C, Cai X, Ye Z, Li H (2015) Genome-wide identification and expression profiling analysis of trihelix gene family in tomato. Biochem Biophys Res Commun 468(4):653–659. https://doi.org/10.1016/j.bbrc.2015.11.010
Ma Z, Liu M, Sun W, Huang L, Wu Q, Bu T, Li C, Chen H (2019) Genome-wide identification and expression analysis of the trihelix transcription factor family in tartary buckwheat (Fagopyrum tataricum). BMC Plant Biol 19(1):344. https://doi.org/10.1186/s12870-019-1957-x
Lam E, Chua NH (1990) GT-1 binding site confers light responsive expression in transgenic tobacco. Science 248(4954):471–474. https://doi.org/10.1126/science.2330508
Green PJ, Kay SA, Chua NH (1987) Sequence-specific interactions of a pea nuclear factor with light-responsive elements upstream of the rbcS-3A gene. EMBO J 6(9):2543–2549. https://doi.org/10.1002/j.1460-2075.1987.tb02542.x
Gao MJ, Lydiate DJ, Li X, Lui H, Gjetvaj B, Hegedus DD, Rozwadowski K (2009) Repression of seed maturation genes by a trihelix transcriptional repressor in Arabidopsis seedlings. Plant Cell 21(1):54–71. https://doi.org/10.1105/tpc.108.061309
Li P, Li Z, Xie G, Zhang J (2021) Trihelix transcription factor ZmThx20 is required for Kernel Development in Maize. Int J Mol Sci 22(22). https://doi.org/10.3390/ijms222212137
Wang Z, Liu Q, Wang H, Zhang H, Xu X, Li C, Yang C (2016) Comprehensive analysis of trihelix genes and their expression under biotic and abiotic stresses in Populus trichocarpa. Sci Rep 6:36274. https://doi.org/10.1038/srep36274
Li B, Jiang S, Yu X, Cheng C, Chen S, Cheng Y, Yuan JS, Jiang D, He P, Shan L (2015) Phosphorylation of trihelix transcriptional repressor ASR3 by MAP KINASE4 negatively regulates Arabidopsis immunity. Plant Cell 27(3):839–856. https://doi.org/10.1105/tpc.114.134809
Liu W, Zhang Y, Li W, Lin Y, Wang C, Xu R, Zhang L (2020) Genome-wide characterization and expression analysis of soybean trihelix gene family. PeerJ 8:e8753. https://doi.org/10.7717/peerj.8753
Xie ZM, Zou HF, Lei G, Wei W, Zhou QY, Niu CF, Liao Y, Tian AG, Ma B, Zhang WK, Zhang JS, Chen SY (2009) Soybean trihelix transcription factors GmGT-2A and GmGT-2B improve plant tolerance to abiotic stresses in transgenic Arabidopsis. PLoS ONE 4(9):e6898. https://doi.org/10.1371/journal.pone.0006898
Xi J, Qiu Y, Du L, Poovaiah BW (2012) Plant-specific trihelix transcription factor AtGT2L interacts with calcium/calmodulin and responds to cold and salt stresses. Plant Sci 185–186:274–280. https://doi.org/10.1016/j.plantsci.2011.11.013
Long J, Xiaoming Y, Dianyuan C, Huan W, Duo C, Jingzhe Z, Jianming L (2020) Expression analysis of trihelix gene family in maize (Zea mays) using bioinformatics. Int J Agric Biology 23:863–868
Namiki M (2007) Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr 47(7):651–673. https://doi.org/10.1080/10408390600919114
Ghotbzadeh Kermani S, Saeidi G, Sabzalian MR, Gianinetti A (2019) Drought stress influenced sesamin and sesamolin content and polyphenolic components in sesame (Sesamum indicum L) populations with contrasting seed coat colors. Food Chem 289:360–368. https://doi.org/10.1016/j.foodchem.2019.03.004
Li D, Dossa K, Zhang Y, Wei X, Wang L, Zhang Y, Liu A, Zhou R, Zhang X (2018) GWAS uncovers Differential Genetic Bases for Drought and Salt Tolerances in Sesame at the Germination Stage. Genes (Basel) 9(2). https://doi.org/10.3390/genes9020087
Wei W, Li D, Wang L, Ding X, Zhang Y, Gao Y, Zhang X (2013) Morpho-anatomical and physiological responses to waterlogging of sesame (Sesamum indicum L). Plant Sci 208:102–111. https://doi.org/10.1016/j.plantsci.2013.03.014
Zhang Y, Li D, Wang Y, Zhou R, Wang L, Zhang Y, Yu J, Gong H, You J, Zhang X (2018) Genome-wide identification and comprehensive analysis of the NAC transcription factor family in Sesamum indicum. PLoS ONE 13(6):e0199262. https://doi.org/10.1371/journal.pone.0199262
Li D, Liu P, Yu J, Wang L, Dossa K, Zhang Y, Zhou R, Wei X, Zhang X (2017) Genome-wide analysis of WRKY gene family in the sesame genome and identification of the WRKY genes involved in responses to abiotic stresses. BMC Plant Biol 17(1):152. https://doi.org/10.1186/s12870-017-1099-y
Wang Y, Zhang Y, Zhou R, Dossa K, Yu J, Li D, Liu A, Mmadi MA, Zhang X, You J (2018) Identification and characterization of the bZIP transcription factor family and its expression in response to abiotic stresses in sesame. PLoS ONE 13(7):e0200850. https://doi.org/10.1371/journal.pone.0200850
Wei M, Liu A, Zhang Y, Zhou Y, Li D, Dossa K, Zhou R, Zhang X, You J (2019) Genome-wide characterization and expression analysis of the HD-Zip gene family in response to drought and salinity stresses in sesame. BMC Genomics 20(1):748. https://doi.org/10.1186/s12864-019-6091-5
Liang JC, Sun J, Yan TX, Le MW, Rao YL, Yan XW, Zhou HY (2021) Genome-wide identification and expression analysis of MATE Gene Family in Sesame. Genomics and Applied Biology 40(02):735–746. https://doi.org/10.13417/j.gab.040.000735
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R (2020) TBtools: an integrative Toolkit developed for interactive analyses of big Biological Data. Mol Plant 13(8):1194–1202. https://doi.org/10.1016/j.molp.2020.06.009
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Li J, Zhang M, Sun J, Mao X, Wang J, Wang J, Liu H, Zheng H, Zhen Z, Zhao H, Zou D (2019) Genome-wide characterization and identification of Trihelix transcription factor and expression profiling in response to Abiotic stresses in Rice (Oryza sativa L). Int J Mol Sci 20(2). https://doi.org/10.3390/ijms20020251
Gil N, Ulitsky I (2020) Regulation of gene expression by cis-acting long non-coding RNAs. Nat Rev Genet 21(2):102–117. https://doi.org/10.1038/s41576-019-0184-5
Wittkopp PJ, Kalay G (2012) Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat Rev Genet 13(1):59–69. https://doi.org/10.1038/nrg3095
Kaplan-Levy RN, Brewer PB, Quon T, Smyth DR (2012) The trihelix family of transcription factors–light, stress and development. Trends Plant Sci 17(3):163–171. https://doi.org/10.1016/j.tplants.2011.12.002
Cui BL, Xu CZ, Liu Y, You DD, Yuan CL (2021) Transcriptome analysis of SlGTL5 in regulation of Tomato Leaf Development. Mol Plant Breed 19(14):4600–4609. https://doi.org/10.13271/j.mpb.019.004600
Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV (2010) The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22(12):4128–4141. https://doi.org/10.1105/tpc.110.078691
Ruiz KA, Pelletier JM, Wang Y, Feng MJ, Behr JS, Ðào TQ, Li B, Kliebenstein D, Harada JJ, Jenik PD (2021) A reevaluation of the role of the ASIL trihelix transcription factors as repressors of the seed maturation program. Plant Direct 5(10):e345. https://doi.org/10.1002/pld3.345
Yang W, Hu J, Behera JR, Kilaru A, Yuan Y, Zhai Y, Xu Y, Xie L, Zhang Y, Zhang Q, Niu L (2021) A Tree Peony Trihelix transcription factor PrASIL1 represses seed oil Accumulation. Front Plant Sci 12:796181. https://doi.org/10.3389/fpls.2021.796181
Li Y, Hu Z, Dong Y, Xie Z (2022) Trihelix Transcriptional factor GhGT26 of cotton enhances salinity tolerance in Arabidopsis. Plants (Basel) 11(20). https://doi.org/10.3390/plants11202694
Park HC, Kim ML, Kang YH, Jeon JM, Yoo JH, Kim MC, Park CY, Jeong JC, Moon BC, Lee JH, Yoon HW, Lee SH, Chung WS, Lim CO, Lee SY, Hong JC, Cho MJ (2004) Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol 135(4):2150–2161. https://doi.org/10.1104/pp.104.041442
Maere S, De Bodt S, Raes J, Casneuf T, Van Montagu M, Kuiper M, Van de Peer Y (2005) Modeling gene and genome duplications in eukaryotes. Proc Natl Acad Sci U S A 102(15):5454–5459. https://doi.org/10.1073/pnas.0501102102
Panchy N, Lehti-Shiu M, Shiu SH (2016) Evolution of gene duplication in plants. Plant Physiol 171(4):2294–2316. https://doi.org/10.1104/pp.16.00523
Freeling M (2009) Bias in plant gene content following different sorts of duplication: tandem, whole-genome, segmental, or by transposition. Annu Rev Plant Biol 60:433–453. https://doi.org/10.1146/annurev.arplant.043008.092122
Liu X, Zhang H, Ma L, Wang Z, Wang K (2020) Genome-wide identification and expression profiling analysis of the Trihelix Gene Family under Abiotic stresses in Medicago truncatula. Genes (Basel) 11(11). https://doi.org/10.3390/genes11111389
Zhang H, Zhu J, Gong Z, Zhu JK (2022) Abiotic stress responses in plants. Nat Rev Genet 23(2):104–119. https://doi.org/10.1038/s41576-021-00413-0
Xu H, Shi X, He L, Guo Y, Zang D, Li H, Zhang W, Wang Y (2018) Arabidopsis thaliana Trihelix transcription factor AST1 mediates salt and osmotic stress tolerance by binding to a Novel AGAG-Box and some GT motifs. Plant Cell Physiol 59(5):946–965. https://doi.org/10.1093/pcp/pcy032
Wang XH, Li QT, Chen HW, Zhang WK, Ma B, Chen SY, Zhang JS (2014) Trihelix transcription factor GT-4 mediates salt tolerance via interaction with TEM2 in Arabidopsis. BMC Plant Biol 14:339. https://doi.org/10.1186/s12870-014-0339-7
Hussain Q, Asim M, Zhang R, Khan R, Farooq S, Wu J (2021) Transcription factors interact with ABA through Gene expression and signaling pathways to Mitigate Drought and salinity stress. Biomolecules 11(8). https://doi.org/10.3390/biom11081159
Luo J, Tang S, Mei F, Peng X, Li J, Li X, Yan X, Zeng X, Liu F, Wu Y, Wu G (2017) BnSIP1-1, a Trihelix Family Gene, mediates abiotic stress tolerance and ABA signaling in Brassica napus. Front Plant Sci 8:44. https://doi.org/10.3389/fpls.2017.00044
Acknowledgements
This study was supported by the China Agriculture Research System (CARS-14) and the Joint Project on Sesame Breeding in Jiangxi Province.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.L. and J.S.; Data curation, Y.Z., J.L., Z.W., T.Y, X.Y., W.W.; Formal analysis, Y.Z., J.L., Z.W.; Writing—original draft preparation, Y.Z.; Writing—review and editing, J.L., Z.W., T.Y. X.Y. W.W., M.L. and J.S. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving in human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1:
Primer sequence for qRT‒PCR;
Table S2
: Cis-acting elements identified in SiTH genes
Table S3
: The predicted cis-elements in the promoter regions of the sesame trihelix genes
Table S4
: Relative Expression profiles of the sesame trihelix genes in different tissues
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Liang, J., Wang, Z. et al. Genome-wide identification and expression analysis of the trihelix transcription factor family in sesame (Sesamum indicum L.) under abiotic stress. Mol Biol Rep 50, 8281–8295 (2023). https://doi.org/10.1007/s11033-023-08640-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08640-w