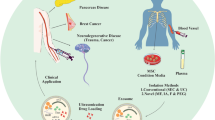
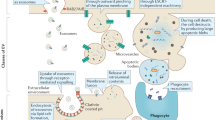
Abstract
Extracellular vesicles (EVs) theranostic potential is under intense investigation. There is a wealth of information highlighting the role that EVs and the secretome play in disease and how these are being utilized for clinical trials and novel therapeutic possibilities. However, understanding of the physiological and pathological roles of EVs remain incomplete. The challenge lies in reaching a consensus concerning standardized quality-controlled isolation, storage, and sample preparation parameters. Interest in circulating EV cargo as diagnostic and prognostic biomarkers is steadily growing. Though promising, various limitations need to be addressed before there can be successful, full-scale therapeutic use of approved EVs. These limitations include obtaining or manufacturing from the appropriate medium (e.g., from bodily fluid or cell culture), loading and isolating EVs, stability, and storage, standardization of processing, and determining potency. This review highlights specific topics, including circulation of abnormal EVs contribute to human disease and the theranostic potential of EVs. Theranostics is defined as a combination of the word’s therapeutics and diagnostics and describes how a specific medicine or technique can function as both. Key findings include, (1) EVs and the secretome are future theranostics which will be utilized as both biomarkers for diagnosis and as therapeutics, (2) basic and translational research supports clinical trials utilizing EVs/secretome, and (3) additional investigation is required to fully unmask the theranostic potential of EVs/secretome in specific diseases and injuries.


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Ela S, Mager I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12(5):347–57. https://doi.org/10.1038/nrd3978
Théry C, Witwer KW, Aikawa E et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750. https://doi.org/10.1080/20013078.2018.1535750
Varcianna A, Myszczynska MA, Castelli LM et al (2019) Micro-RNAs secreted through astrocyte-derived extracellular vesicles cause neuronal network degeneration in C9orf72 ALS. EBioMedicine 40:626–635. https://doi.org/10.1016/j.ebiom.2018.11.067
Cheng Y, Wang X, Yang J et al (2012) A translational study of urine miRNAs in acute myocardial infarction. J Mol Cell Cardiol 53(5):668–676. https://doi.org/10.1016/j.yjmcc.2012.08.010
Cohn W, Melnik M, Huang C et al (2021) Multi-omics analysis of microglial extracellular vesicles from human Alzheimer’s disease brain tissue reveals disease-associated signatures. Front Pharmacol. 12:766082. https://doi.org/10.3389/fphar.2021.766082
Paganini C, Capasso Palmiero U, Pocsfalvi G, Touzet N, Bongiovanni A, Arosio P (2019) Scalable production and isolation of extracellular vesicles: available sources and lessons from current industrial bioprocesses. Biotechnol J 14(10):e1800528. https://doi.org/10.1002/biot.201800528
Cheng A, Choi D, Lora M, Shum-Tim D, Rak J, Colmegna I (2020) Human multipotent mesenchymal stromal cells cytokine priming promotes RAB27B-regulated secretion of small extracellular vesicles with immunomodulatory cargo. Stem Cell Res Ther 11(1):539. https://doi.org/10.1186/s13287-020-02050-6
Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S (2014) Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 14(11):1891–1900. https://doi.org/10.1039/c4lc00136b
Fang X, Chen C, Liu B et al (2021) A magnetic bead-mediated selective adsorption strategy for extracellular vesicle separation and purification. Acta Biomater 124:336–347. https://doi.org/10.1016/j.actbio.2021.02.004
Deregibus MC, Figliolini F, D’Antico S et al (2016) Charge-based precipitation of extracellular vesicles. Int J Mol Med 38(5):1359–1366. https://doi.org/10.3892/ijmm.2016.2759
Zhang J, Nguyen LTH, Hickey R et al (2021) Immunomagnetic sequential ultrafiltration (iSUF) platform for enrichment and purification of extracellular vesicles from biofluids. Sci Rep 11(1):8034. https://doi.org/10.1038/s41598-021-86910-y
Gholizadeh S, Shehata Draz M, Zarghooni M et al (2017) Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: current status and future directions. Biosens Bioelectron 91:588–605. https://doi.org/10.1016/j.bios.2016.12.062
Chen C, Skog J, Hsu CH et al (2010) Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 10(4):505–511. https://doi.org/10.1039/b916199f
Gyorgy B, Szabo TG, Pasztoi M et al (2011) Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68(16):2667–2688. https://doi.org/10.1007/s00018-011-0689-3
Liu F, Vermesh O, Mani V et al (2017) The exosome total isolation chip. ACS Nano 11(11):10712–10723. https://doi.org/10.1021/acsnano.7b04878
Gardiner C, Di Vizio D, Sahoo S et al (2016) Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles 5:32945. https://doi.org/10.3402/jev.v5.32945
Nwokwu CD, Ishraq Bari SM, Hutson KH, Brausell C, Nestorova GG (2022) ExoPRIME: solid-phase immunoisolation and OMICS analysis of surface-marker-specific exosomal subpopulations. Talanta 236:122870. https://doi.org/10.1016/j.talanta.2021.122870
Chen Y, Zhu Q, Cheng L et al (2021) Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods 18(2):212–218. https://doi.org/10.1038/s41592-020-01034-x
Vandergriff A, Huang K, Shen D et al (2018) Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 8(7):1869–1878. https://doi.org/10.7150/thno.20524
Hoshino A, Costa-Silva B, Shen TL et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527(7578):329–335. https://doi.org/10.1038/nature15756
Laulagnier K, Javalet C, Hemming FJ et al (2018) Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci 75(4):757–773. https://doi.org/10.1007/s00018-017-2664-0
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ (2018) Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol 15(10):617–638. https://doi.org/10.1038/s41571-018-0036-9
Namee NM, O’Driscoll L (2018) Extracellular vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev Cancer 1870(2):123–136. https://doi.org/10.1016/j.bbcan.2018.07.003
Stark TR, Davidson NL, Cannon JW et al (2022) Battlefield pain summit 2022: expert consensus statements. J Trauma Acute Care Surg 93:S12–S15. https://doi.org/10.1097/TA.0000000000003711
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345. https://doi.org/10.1038/nbt.1807
Cooper JM, Wiklander PB, Nordin JZ et al (2014) Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord 29(12):1476–1485. https://doi.org/10.1002/mds.25978
Didiot MC, Hall LM, Coles AH et al (2016) Exosome-mediated delivery of hydrophobically modified siRNA for huntingtin mRNA silencing. Mol Ther 24(10):1836–1847. https://doi.org/10.1038/mt.2016.126
Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ (2016) Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci USA 113(1):170–175. https://doi.org/10.1073/pnas.1522297113
Huang S, Ge X, Yu J et al (2018) Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 32(1):512–528. https://doi.org/10.1096/fj.201700673R
Ruppert KA, Nguyen TT, Prabhakara KS et al (2018) Human mesenchymal stromal cell-derived extracellular vesicles modify microglial response and improve clinical outcomes in experimental spinal cord injury. Sci Rep. 8(1):480. https://doi.org/10.1038/s41598-017-18867-w
Grange C, Skovronova R, Marabese F, Bussolati B (2019) Stem cell-derived extracellular vesicles and kidney regeneration. Cells. https://doi.org/10.3390/cells8101240
Bruno S, Grange C, Deregibus MC et al (2009) Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20(5):1053–1067. https://doi.org/10.1681/ASN.2008070798
Cabral J, Ryan AE, Griffin MD, Ritter T (2018) Extracellular vesicles as modulators of wound healing. Adv Drug Deliv Rev 129:394–406. https://doi.org/10.1016/j.addr.2018.01.018
Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V (2019) Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. https://doi.org/10.3390/cells8121605
Cosenza S, Ruiz M, Maumus M, Jorgensen C, Noel D (2017) Pathogenic or therapeutic extracellular vesicles in rheumatic diseases: role of mesenchymal stem cell-derived vesicles. Int J Mol Sci. https://doi.org/10.3390/ijms18040889
Khatri M, Richardson LA, Meulia T (2018) Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther 9(1):17. https://doi.org/10.1186/s13287-018-0774-8
Lai CP, Mardini O, Ericsson M et al (2014) Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8(1):483–494. https://doi.org/10.1021/nn404945r
Kevadiya BD, Woldstad C, Ottemann BM et al (2018) Multimodal theranostic nanoformulations permit magnetic resonance bioimaging of antiretroviral drug particle tissue-cell biodistribution. Theranostics 8(1):256–276. https://doi.org/10.7150/thno.22764
NIH U.S. National Library of Medicine. ClinicalTrials.gov. 2020. https://clinicaltrials.gov/. Accessed 15 Oct 2020
Administration USFD. Consumer Alert on Regenerative Medicine Products Including Stem Cells and Exosomes. Updated 22 JULY 2020, https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/consumer-alert-regenerative-medicine-products-including-stem-cells-and-exosomes. Accessed 15 Oct 2020
Fais S, O’Driscoll L, Borras FE et al (2016) Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano 10(4):3886–3899. https://doi.org/10.1021/acsnano.5b08015
Ko J, Bhagwat N, Yee SS et al (2017) Combining machine learning and nanofluidic technology to diagnose pancreatic cancer using exosomes. ACS Nano 11(11):11182–11193. https://doi.org/10.1021/acsnano.7b05503
Quiroz-Baez R, Hernandez-Ortega K, Martinez-Martinez E (2020) Insights into the proteomic profiling of extracellular vesicles for the identification of early biomarkers of neurodegeneration. Front Neurol 11:580030. https://doi.org/10.3389/fneur.2020.580030
Wolf P (1967) The nature and significance of platelet products in human plasma. Br J Haematol 13(3):269–288. https://doi.org/10.1111/j.1365-2141.1967.tb08741.x
Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C et al (2011) Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2:282. https://doi.org/10.1038/ncomms1285
Goloviznina NA, Verghese SC, Yoon YM, Taratula O, Marks DL, Kurre P (2017) Mesenchymal stromal cell-derived extracellular vesicles promote myeloid-biased multipotent hematopoietic progenitor expansion via Toll-like receptor engagement. J Biol Chem 292(8):3541. https://doi.org/10.1074/jbc.A116.745653
Zwaal RF, Schroit AJ (1997) Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 89(4):1121–1132
Xin H, Li Y, Liu Z et al (2013) MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31(12):2737–2746. https://doi.org/10.1002/stem.1409
Hayon Y, Shai E, Varon D, Leker RR (2012) The role of platelets and their microparticles in rehabilitation of ischemic brain tissue. CNS Neurol Disord Drug Targets 11(7):921–925. https://doi.org/10.2174/1871527311201070921
Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R (2008) Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull 31(6):1059–1062. https://doi.org/10.1248/bpb.31.1059
Winck FV, Prado Ribeiro AC, Ramos Domingues R et al (2015) Insights into immune responses in oral cancer through proteomic analysis of saliva and salivary extracellular vesicles. Sci Rep 5:16305. https://doi.org/10.1038/srep16305
Cheng Y, Pereira M, Raukar N et al (2019) Potential biomarkers to detect traumatic brain injury by the profiling of salivary extracellular vesicles. J Cell Physiol 234(8):14377–14388. https://doi.org/10.1002/jcp.28139
Cao Z, Wu Y, Liu G et al (2019) alpha-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson’s disease. Neurosci Lett 696:114–120. https://doi.org/10.1016/j.neulet.2018.12.030
Aqrawi LA, Galtung HK, Vestad B et al (2017) Identification of potential saliva and tear biomarkers in primary Sjogren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res Ther 19(1):14. https://doi.org/10.1186/s13075-017-1228-x
Pisitkun T, Shen RF, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101(36):13368–13373. https://doi.org/10.1073/pnas.0403453101
Miyazawa Y, Mikami S, Yamamoto K et al (2018) AQP2 in human urine is predominantly localized to exosomes with preserved water channel activities. Clin Exp Nephrol 22(4):782–788. https://doi.org/10.1007/s10157-018-1538-6
Street JM, Birkhoff W, Menzies RI, Webb DJ, Bailey MA, Dear JW (2011) Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J Physiol 589(Pt 24):6119–6127. https://doi.org/10.1113/jphysiol.2011.220277
Feng Y, Lv LL, Wu WJ et al (2018) Urinary exosomes and exosomal CCL2 mRNA as Biomarkers of active histologic injury in IgA nephropathy. Am J Pathol 188(11):2542–2552. https://doi.org/10.1016/j.ajpath.2018.07.017
Aalberts M, Sostaric E, Wubbolts R et al (2013) Spermatozoa recruit prostasomes in response to capacitation induction. Biochim Biophys Acta 1834(11):2326–2335. https://doi.org/10.1016/j.bbapap.2012.08.008
Frenette G, Lessard C, Madore E, Fortier MA, Sullivan R (2003) Aldose reductase and macrophage migration inhibitory factor are associated with epididymosomes and spermatozoa in the bovine epididymis. Biol Reprod 69(5):1586–1592. https://doi.org/10.1095/biolreprod.103.019216
Barraud-Lange V, Naud-Barriant N, Bomsel M, Wolf JP, Ziyyat A (2007) Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J 21(13):3446–3449. https://doi.org/10.1096/fj.06-8035hyp
Machtinger R, Laurent LC, Baccarelli AA (2016) Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update Mar-Apr 22(2):182–193. https://doi.org/10.1093/humupd/dmv055
Lyu Y, Kaddour H, Kopcho S et al (2019) Human immunodeficiency virus (HIV) infection and use of illicit substances promote secretion of semen exosomes that enhance monocyte adhesion and induce actin reorganization and chemotactic migration. Cells. https://doi.org/10.3390/cells8091027
Friedman H, Newton C, Klein TW (2003) Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev 16(2):209–219. https://doi.org/10.1128/CMR.16.2.209-219.2003
Welch JL, Madison MN, Margolick JB et al (2017) Effect of prolonged freezing of semen on exosome recovery and biologic activity. Sci Rep 7:45034. https://doi.org/10.1038/srep45034
Ayaz A, Houle E, Pilsner JR (2021) Extracellular vesicle cargo of the male reproductive tract and the paternal preconception environment. Syst Biol Reprod Med 67(2):103–111. https://doi.org/10.1080/19396368.2020.1867665
Welch JL, Kaddour H, Schlievert PM, Stapleton JT, Okeoma CM (2018) Semen exosomes promote transcriptional silencing of HIV-1 by disrupting NF-kappaB/Sp1/Tat circuitry. J Virol. https://doi.org/10.1128/JVI.00731-18
Madison MN, Jones PH, Okeoma CM (2015) Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology 482:189–201. https://doi.org/10.1016/j.virol.2015.03.040
Gustafson CM, Shepherd AJ, Miller VM, Jayachandran M (2015) Age- and sex-specific differences in blood-borne microvesicles from apparently healthy humans. Biol Sex Differ 6:10. https://doi.org/10.1186/s13293-015-0028-8
Toth B, Nikolajek K, Rank A et al (2007) Gender-specific and menstrual cycle dependent differences in circulating microparticles. Platelets 18(7):515–521. https://doi.org/10.1080/09537100701525843
Bammert TD, Hijmans JG, Kavlich PJ et al (2017) Influence of sex on the number of circulating endothelial microparticles and microRNA expression in middle-aged adults. Exp Physiol 102(8):894–900. https://doi.org/10.1113/EP086359
Dekker M, Waissi F, van Bennekom J et al (2020) Extracellular Vesicle cystatin c is associated with unstable angina in troponin negative patients with acute chest pain. PLoS One 15(8):e0237036. https://doi.org/10.1371/journal.pone.0237036
Raval AP, Martinez CC, Mejias NH, de Rivero Vaccari JP (2019) Sexual dimorphism in inflammasome-containing extracellular vesicles and the regulation of innate immunity in the brain of reproductive senescent females. Neurochem Int 127:29–37. https://doi.org/10.1016/j.neuint.2018.11.018
Hunter LW, Jayachandran M, Miller VM (2019) Sex differences in the expression of cell adhesion molecules on microvesicles derived from cultured human brain microvascular endothelial cells treated with inflammatory and thrombotic stimuli. Biol Sex Differ 10(1):26. https://doi.org/10.1186/s13293-019-0241-y
Ibanez F, Urena-Peralta JR, Costa-Alba P et al (2020) Circulating MicroRNAs in extracellular vesicles as potential biomarkers of alcohol-induced neuroinflammation in adolescence: gender differences. Int J Mol Sci. https://doi.org/10.3390/ijms21186730
Sun B, Fernandes N, Pulliam L (2019) Profile of neuronal exosomes in HIV cognitive impairment exposes sex differences. AIDS 33(11):1683–1692. https://doi.org/10.1097/QAD.0000000000002272
Ho DH, Yi S, Seo H, Son I, Seol W (2014) Increased DJ-1 in urine exosome of Korean males with Parkinson’s disease. Biomed Res Int 2014:704678. https://doi.org/10.1155/2014/704678
Crotti A, Sait HR, McAvoy KM et al (2019) BIN1 favors the spreading of Tau via extracellular vesicles. Sci Rep 9(1):9477. https://doi.org/10.1038/s41598-019-45676-0
Jung H, Park H, Choi Y et al (2018) Sexually dimorphic behavior, neuronal activity, and gene expression in Chd8-mutant mice. Nat Neurosci 21(9):1218–1228. https://doi.org/10.1038/s41593-018-0208-z
Kolhe R, Hunter M, Liu S et al (2017) Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep 7(1):2029. https://doi.org/10.1038/s41598-017-01905-y
Kolhe R, Owens V, Sharma A et al (2020) Sex-specific differences in extracellular vesicle protein cargo in synovial fluid of patients with osteoarthritis. Life (Basel). https://doi.org/10.3390/life10120337
Ben-Dov IZ, Whalen VM, Goilav B, Max KE, Tuschl T (2016) Cell and microvesicle urine microrna deep sequencing profiles from healthy individuals: observations with potential impact on biomarker studies. PLoS One. 11(1):e0147249. https://doi.org/10.1371/journal.pone.0147249
Turco AE, Lam W, Rule AD et al (2016) Specific renal parenchymal-derived urinary extracellular vesicles identify age-associated structural changes in living donor kidneys. J Extracell Vesicles 5:29642. https://doi.org/10.3402/jev.v5.29642
Jayachandran M, Lugo G, Heiling H, Miller VM, Rule AD, Lieske JC (2015) Extracellular vesicles in urine of women with but not without kidney stones manifest patterns similar to men: a case control study. Biol Sex Differ 6:2. https://doi.org/10.1186/s13293-015-0021-2
RamachandraRao SP, Matthias MA, Kokoy-Mondragon C et al (2015) Proteomic analysis of urine exosomes reveals renal tubule response to leptospiral colonization in experimentally infected rats. PLoS Negl Trop Dis 9(3):e0003640. https://doi.org/10.1371/journal.pntd.0003640
Lansford KA, Shill DD, Dicks AB, Marshburn MP, Southern WM, Jenkins NT (2016) Effect of acute exercise on circulating angiogenic cell and microparticle populations. Exp Physiol 101(1):155–167. https://doi.org/10.1113/EP085505
Shill DD, Lansford KA, Hempel HK, Call JA, Murrow JR, Jenkins NT (2018) Effect of exercise intensity on circulating microparticles in men and women. Exp Physiol 103(5):693–700. https://doi.org/10.1113/EP086644
Rigamonti AE, Bollati V, Pergoli L et al (2020) Effects of an acute bout of exercise on circulating extracellular vesicles: tissue-, sex-, and BMI-related differences. Int J Obes (Lond) 44(5):1108–1118. https://doi.org/10.1038/s41366-019-0460-7
Silver JL, Alexander SE, Dillon HT, Lamon S, Wadley GD (2020) Extracellular vesicular miRNA expression is not a proxy for skeletal muscle miRNA expression in males and females following acute, moderate intensity exercise. Physiol Rep 8(16):e14520. https://doi.org/10.14814/phy2.14520
Gomez-Molina C, Sandoval M, Henzi R et al (2019) Small extracellular vesicles in rat serum contain astrocyte-derived protein biomarkers of repetitive stress. Int J Neuropsychopharmacol 22(3):232–246. https://doi.org/10.1093/ijnp/pyy098
Beninson LA, Brown PN, Loughridge AB et al (2014) Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (Hsp72) and microRNA (miR-142–5p and miR-203). PLoS One 9(9):e108748. https://doi.org/10.1371/journal.pone.0108748
Choi J, Kim YK, Han PL (2019) Extracellular vesicles derived from lactobacillus plantarum Increase BDNF expression in cultured hippocampal neurons and produce antidepressant-like effects in mice. Exp Neurobiol 28(2):158–171. https://doi.org/10.5607/en.2019.28.2.158
Saeedi S, Israel S, Nagy C, Turecki G (2019) The emerging role of exosomes in mental disorders. Transl Psychiatry 9(1):122. https://doi.org/10.1038/s41398-019-0459-9
Banigan MG, Kao PF, Kozubek JA et al (2013) Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS One 8(1):e48814. https://doi.org/10.1371/journal.pone.0048814
Amoah SK, Rodriguez BA, Logothetis CN et al (2020) Exosomal secretion of a psychosis-altered miRNA that regulates glutamate receptor expression is affected by antipsychotics. Neuropsychopharmacology 45(4):656–665. https://doi.org/10.1038/s41386-019-0579-1
Mansur RB, Delgado-Peraza F, Subramaniapillai M et al (2020) Extracellular Vesicle Biomarkers Reveal Inhibition of Neuroinflammation by Infliximab in Association with Antidepressant Response in Adults with Bipolar Depression. Cells. https://doi.org/10.3390/cells9040895
Acknowledgements
Not applicable.
Disclaimer
The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, DoD, or the U.S. Government.
Military Relevance
Future Combat Casualty Care may indeed utilize EV and/or secretome based therapeutics to treat wounded Soldiers on the battlefield. EV/secretome theranostics could potentially be used in all aspects of medical care that support the overall readiness of the Soldier.
Funding
This research was supported by Combat Casualty Care Research Program (CCCRP, MR190014).
Author information
Authors and Affiliations
Contributions
Dr. NS coordinated all author’s sections, edited all sections, and wrote section “Conclusions” and made Fig. 2. Dr. AM wrote “EV Structure and Content” section edited manuscript, made supplemental figure, and made Fig. 1. MH wrote “Biomarker potential of EVs” section. Dr. HL wrote “Therapeutic potential of EVs” section. JS wrote section “Contribution to mental health”. Dr. Averitt wrote “Sex Differences in EVs” section. Dr. SN wrote “EV isolation technique” section. JS and JM wrote “EVs contribution to mental health” section. Dr. Stark edited the manuscript extensively.
Corresponding author
Ethics declarations
Competing interests
The authors have no non-financial or financial interests to declare.
Ethical approval and consent to participate
All authors consent to participate. All figures are licensed to publish.
Consent for publication
All material is consented to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammadipoor, A., Hershfield, M.R., Linsenbardt, H.R. et al. Biological function of Extracellular Vesicles (EVs): a review of the field. Mol Biol Rep 50, 8639–8651 (2023). https://doi.org/10.1007/s11033-023-08624-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08624-w