Abstract
Background
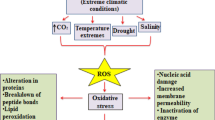
Climate change has had a tremendous impact on the environment in general as well as agricultural crops grown in these situations as time passed. Agricultural production of crops is less suited and of lower quality due to disturbances in plant metabolism brought on by sensitivity to environmental stresses, which are brought on by climate change. Abiotic stressors that are specific to climate change, including as drought, extremes in temperature, increasing CO2, waterlogging from heavy rain, metal toxicity, and pH changes, are known to negatively affect an array of species. Plants adapt to these challenges by undergoing genome-wide epigenetic changes, which are frequently accompanied by differences in transcriptional gene expression. The sum of a cell’s biochemical modifications to its nuclear DNA, post-translational modifications to histones, and variations in the synthesis of non-coding RNAs is called an epigenome. These modifications frequently lead to variations in gene expression that occur without any alteration in the underlying base sequence.
Epigenetic mechanisms and marks
The methylation of homologous loci by three different modifications—genomic (DNA methylation), chromatin (histone modifications), and RNA-directed DNA methylation (RdDM)-could be regarded as epigenetic mechanisms that control the regulation of differential gene expression. Stresses from the environment cause chromatin remodelling, which enables plant cells to adjust their expression patterns temporarily or permanently.
Epigenomics’ consequences for genome stability and gene expression
DNA methylation affects gene expression in response to abiotic stressors by blocking or suppressing transcription. Environmental stimuli cause changes in DNA methylation levels, either upward in the case of hypermethylation or downward in the case of hypomethylation. The type of stress response that occurs as a result also affects the degree of DNA methylation alterations. Stress is also influenced by DRM2 and CMT3 methylating CNN, CNG, and CG. Both plant development and stress reactions depend on histone changes. Gene up-regulation is associated with histone tail phosphorylation, ubiquitination, and acetylation, while gene down-regulation is associated with de-acetylation and biotinylation. Plants undergo a variety of dynamic changes to histone tails in response to abiotic stressors. The relevance of these transcripts against stress is highlighted by the accumulation of numerous additional antisense transcripts, a source of siRNAs, caused by abiotic stresses. The study highlights the finding that plants can be protected from a range of abiotic stresses by epigenetic mechanisms such DNA methylation, histone modification, and RNA-directed DNA methylation.
Transgenerational inheritance and sources of epigenetic variation
Stress results in the formation of epialleles, which are either transient or enduring epigenetic stress memory in plants. After the stress is gone, the stable memory is kept for the duration of the plant’s remaining developmental cycles or passed on to the next generations, leading to plant evolution and adaptability. The bulk of epigenetic changes brought on by stress are temporary and return to normal after the stress has passed. Some of the modifications, however, might be long-lasting and transmitted across mitotic or even meiotic cell divisions. Epialleles often have genetic or non-genetic causes. Epialleles can arise spontaneously due to improper methylation state maintenance, short RNA off-target effects, or other non-genetic causes. Developmental or environmental variables that influence the stability of epigenetic states or direct chromatin modifications may also be non-genetic drivers of epigenetic variation. Transposon insertions that change local chromatin and structural rearrangements, such copy number changes that are genetically related or unrelated, are two genetic sources of epialleles.
Epigenomics in crop improvement
To include epigenetics into crop breeding, it is necessary to create epigenetic variation as well as to identify and evaluate epialleles. Epigenome editing or epi-genomic selection may be required for epiallele creation and identification. In order to combat the challenges given by changing environments, these epigenetic mechanisms have generated novel epialleles that can be exploited to develop new crop types that are more climate-resilient. Numerous techniques can be used to alter the epigenome generally or at specific target loci in order to induce the epigenetic alterations necessary for crop development. Technologies like CRISPR/Cas9 and dCas, which have recently advanced, have opened up new avenues for the study of epigenetics. Epialleles could be employed in epigenomics-assisted breeding in addition to sequence-based markers for crop breeding.
Conclusions and future prospectus
A few of the exciting questions that still need to be resolved in the area of heritable epigenetic variation include a better understanding of the epigenetic foundation of characteristics, the stability and heritability of epialleles, and the sources of epigenetic variation in crops. Investigating long intergenic non-coding RNAs (lincRNAs) as an epigenetic process might open up a new path to understanding crop plant’s ability to withstand abiotic stress. For many of these technologies and approaches to be more applicable and deployable at a lower cost, technological breakthroughs will also be necessary. Breeders will probably need to pay closer attention to crop epialleles and how they can affect future responses to climate changes. The development of epialleles suitable for particular environmental circumstances may be made possible by creating targeted epigenetic changes in pertinent genes and by comprehending the molecular underpinnings of trans generational epigenetic inheritance. More research on a wider variety of plant species is required in order to fully comprehend the mechanisms that produce and stabilise epigenetic variation in crops. In addition to a collaborative and multidisciplinary effort by researchers in many fields of plant science, this will require a greater integration of the epigenomic data gathered in many crops. Before it may be applied generally, more study is required.




Similar content being viewed by others
References
Andy P (2016) Abiotic stress tolerance in plants. Plant Sci 7:1–9
Hirayama T, Shinozaki K (2010) Research on plant abiotic stress responses in the post-genome era: past, present and future. The Plant J 61:1041–1052
Rosenzweig C, Elliott J, Deryng D, Ruane AC, Müller C, Arneth A (2014) Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc Natl Acad Sci USA 3268–3273
Wheeler T, Von Braun J (2013) Climate change impacts on global food security. Sci Direct 341:508–513
Rejeb IB, Pastor V, Mauch-Mani B (2014) Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plant Cell Environ 3:458–475
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11(1):15–19
Compant S, Van Der Heijden MG, Sessitsch A (2010) Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol Ecol 73(2):197–214
Ashraf M, Foolad MR (2005) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377
Gehring M, Henikof S (2007) DNA methylation dynamics in plant genomes. Biochim Biophys Acta 1769:276–286
Chinnusamy V, Zhu JK (2009) Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12:133–139
Waddington CH (1942) Canalization of development and the inheritance of acquired characters. Nature 150(3811):563–565
Tsaftaris AS, Polidoros AN, Kapazoglou A, Tani E, Kovacevic NM (2007) Epigenetics and plant breeding. Plant Breed Rev 30:49–178
Bonasio R, Tu S, Reinberg D (2010) Molecular signals of epigenetic states. Science 330:612–616
Springer NM, Schmitz RJ (2017) Exploiting induced and natural epigenetic variation for crop improvement. Nat Rev Genet 18(9):563–575. https://doi.org/10.1038/nrg.2017.45
Hidetoshi S, Kazuo T, Tatsuo K, Taisuke N (2012) DNA methylation in plants: relationship to small RNAs and histone modifications, and functions in transposon inactivation. Plant Cell Physiol 53(5):766–784
Karlsson M, Weber W, Fussenegger M (2011) De novo design and construction of an inducible gene expression system in mammalian cells. Methods Enzymol 497:239–253
Zhang H, Lang Z, Zhu JK (2018) Dynamics and function of DNA methylation in plants. Nat Rev Mol Cell Biol 19:489–506
Zemach A, Kim MY, Hsieh PH, Coleman-Derr D, Eshed-Williams L, Thao K, Harmer SL, Zilberman D (2013) The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell 153:193–205
Zhu JK (2009) Active DNA methylation mediated by DNA glycosylases. Annu Rev Genet 43:143–166
Ibarra CA, Feng X, Schoft VK, Hsieh TF, Uzawa R, Rodrigues JA et al (2012) Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science 337(6100):1360–1364
Penterman J, Uzawa R, Fischer RL (2007) Genetic interactions between DNA demethylation and methylation in Arabidopsis. Plant Physiol 145(4):1549–1557
Law JA, Jacobsen SE (2010) Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 11(3):204–220
Xu J, Wang Q, Freeling M et al (2017) Natural antisense transcripts are significantly involved in regulation of drought stress in maize. Nucleic Acids Res 45:5126–5141
Forestan C, Aiese Cigliano R, Farinati S, Lunardon A, Sanseverino W, Varotto S (2016) Stress- induced and epigenetic-mediated maize transcriptome regulation study by means of transcriptome reannotation and differential expression analysis. Sci Rep 6:30446
Wang N, Ku LX, Chen YH, Wang W (2015) Comparative proteomic analysis of leaves between photoperiod-sensitive and photoperiod-insensitive maize inbred seedlings under long day treatments. Acta Physiol Plant 37:1705
Hou H, Zhao L, Zheng X, Gautam M, Yue M, Hou J, Chen Z, Wang P, Li L (2019) Dynamic changes in histone modification are associated with upregulation of Hsf and rRNA genes during heat stress in maize seedlings. Protoplasma 256:1245–1256
Steward N, Kusano T, Sano H (2000) Expression of ZmMET1, a gene encoding a DNA methyl transferase from maize, is associated not only with DNA replication in actively proliferating cells, but also with altered DNA methylation status in cold-stressed quiescent cells. Nucleic Acids Res 28:3250–3259
Steward N, Ito M, Yamaguchi Y, Koizumi N, Sano H (2002) Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. J Biol Chem 277:37741–37746
Hu X, Wu X, Li C, Lu M, Liu T, Wang Y, Wang W (2012) Abscisic acid refines the synthesis of chloroplast proteins in maize (Zea mays) in response to drought and light. PLoS ONE 7:e49500
Hu XL, Lu MH, Li CH et al (2011) Differential expression of proteins in maize roots in response to abscisic acid and drought. Acta Physiol Plant 33:2437–2446
Wang Y, Li H, Sun Q, Yao Y (2016) Characterization of small RNAs derived from tRNAs, rRNAs and snoRNAs and their response to heat stress in wheat seedlings. PLoS ONE 11:e0150933
Zhong L, Xu YH, Wang JB (2009) DNA-methylation changes induced by salt stress in wheat Triticum aestivum. Afr J Biotechnol 8:6201–6207
Kumar S, Beena AS, Awana M, Singh A (2017) Physiological, biochemical, epigenetic and molecular analyses of wheat (Triticum aestivum) genotypes with contrasting salt tolerance. Front Plant Sci 8:1151
Kantar M, Unver T, Budak H (2010) Regulation of barley miRNAs upon dehydration stress correlated with target gene expression. Funct Integr Genomics 10:493–507
Papaefthimiou D, Tsaftaris A (2012) Characterization of a drought inducible trithorax-like H3K4 methyltransferase from barley. Biol Plant 56:683–692
Surdonja K, Eggert K, Hajirezaei MR, Harshavardhan V, Seiler C, von Wirén N, Sreenivasulu N, Kuhlmann M (2017) Increase of DNA methylation at the HvCKX2.1 promoter by terminal drought stress in barley. Epigenomes 1:9
Temel A, Janack B, Humbeck K (2017) Drought stress-related physiological changes and histone modifications in barley primary leaves at HSP17 gene. Agronomy 7:43
Wang W, Pan YJ, Zhao XQ, Dwivedi D, Zhu LH, Ali J, Fu BY, Li ZK (2011) Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.). J Exp Bot 62:1951–1960
Wang W, Zhao X, Pan Y, Zhu L, Fu B, Li Z (2011) DNA methylation changes detected by methylation-sensitive amplified polymorphism in two contrasting rice genotypes under salt stress. J Genet Genomics 38:419–424
Gayacharan A, Joel AJ (2013) Epigenetic responses to drought stress in rice (Oryza sativa L.). Physiol Mol Biol Plants 19:379–387
Mutum RD, Balyan SC, Kansal S, Agarwal P, Kumar S, Kumar M, Raghuvanshi S (2013) Evolution of variety-specific regulatory schema for expression of osa-miR408 in indica rice varieties under drought stress. FEBS J 280:1717–1730
Karan R, DeLeon T, Biradar H, Subudhi PK (2012) Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PLoS ONE 7:e40203
Zhu N, Cheng S, Liu X, Du H, Dai M, Zhou DX, Yang W, Zhao Y (2015) The R2R3-type MYB gene OsMYB91 has a function in coordinating plant growth and salt stress tolerance in rice. Plant Sci 236:146–156
Ferreira LJ, Donoghue MT, Barros P, Saibo NJ, Santos AP, Oliveira MM (2019) Uncovering differentially methylated regions (DMRs) in a salt-tolerant rice variety under stress: one step towards new regulatory regions for enhanced salt tolerance. Epigenomes 3:4
Kulcheski FR, de Oliveira LF, Molina LG et al (2011) Identification of novel soybean microRNAs involved in abiotic and biotic stresses. BMC Genomics 12:307
Sosa-Valencia G, Palomar M, Covarrubias AA, Reyes JL (2017) The legume miR1514a modulates a NAC transcription factor transcript to trigger phasiRNA formation in response to drought. J Exp Bot 68:2013–2026
Hossain MS, Kawakatsu T, Kim KD et al (2017) Divergent cytosine DNA methylation patterns in single-cell, soybean root hairs. New Phytol 214:808–819
Labra M, Ghiani A, Citterio S, Sgorbati S, Sala F, Vannini C, Ruffini-Castiglione M, Bracale M (2002) Analysis of cytosine methylation pattern in response to water deficit in pea root tips. Plant Biol 4:694–699
Hajyzadeh M, Turktas M, Khawar KM, Unver T (2015) miR408 overexpression causes increased drought tolerance in chickpea. Gene 555:186–193
Khandal H, Parween S, Roy R, Meena MK, Chattopadhyay D (2017) MicroRNA profiling provides insights into post-transcriptional regulation of gene expression in chickpea root apex under salinity and water deficiency. Sci Rep 7:4632
Shui XR, Chen ZW, Li JX (2013) MicroRNA prediction and its function in regulating drought- related genes in cowpea. Plant Sci 210:25–35
De la Rosa C, Covarrubias AA, Reyes JL (2019) A dicistronic precursor encoding miR398 and the legume-specific miR2119 coregulates CSD1 and ADH1 mRNAs in response to water deficit. Plant Cell Environ 42:133–144
Abid G, Mingeot D, Muhovski Y et al (2017) Analysis of DNA methylation patterns associated with stress response in faba bean (Vicia faba L.) using methylation-sensitive amplification polymorphism (MSAP). Environ Exp Bot 142:34–44
Arshad M, Gruber MY, Hannoufa A (2018) Transcriptome analysis of microRNA156 overexpression alfalfa roots under drought stress. Sci Rep 8:9363
Gao G, Li J, Li H, Li F, Xu K, Yan G, Chen B, Qiao J, Wu X (2014) Comparison of the heat stress induced variations in DNA methylation between heat-tolerant and heat-sensitive rapeseed seedlings. Breed Sci 64:125–133
Marconi G, Pace R, Traini A, Raggi L, Lutts S, Chiusano M, Guiducci M, Falcinelli M, Benincasa P, Albertini E (2013) Use of MSAP markers to analyse the effects of salt stress on DNA methylation in rapeseed (Brassica napus var. oleifera). PLoS ONE 8:e75597
Shea DJ, Nishida N, Takada S, Itabashi E, Takahashi S, Akter A et al (2019) Long noncoding RNAs in Brassica rapa L following vernalization. Sci Rep 9:9302
Benoit M, Drost HG, Catoni M, Gouil Q, Lopez-Gomollon S, Baulcombe D, Paszkowski J (2019) Environmental and epigenetic regulation of Rider retrotransposons in tomato. PLoS Genet 15:e1008370
González RM, Ricardi MM, Iusem ND (2011) Atypical epigenetic mark in an atypical location: cytosine methylation at asymmetric (CNN) sites within the body of a non-repetitive tomato gene. BMC Plant Biol 11:94
Huang W, Xian Z, Hu G, Li Z (2016) SlAGO4A, a core factor of RNA directed DNA methylation (RdDM) pathway, plays an important role under salt and drought stress in tomato. Mol Breed 36(3):28
Zhang B, Tieman DM, Jiao C, Xu Y, Chen K, Fei Z, Giovannoni JJ, Klee HJ (2016) Chilling- induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc Natl Acad Sci USA 113:12580–12585
Yolcu S, Ozdemir F, Güler A, Bor M (2016) Histone acetylation infuences the transcriptional activation of POX in Beta vulgaris L. and Beta maritima L. under salt stress. Plant Physiol Biochem 100:37–46
Zheng Y, Ding Y, Sun X, Xie S, Wang D, Liu X, Zhou DX (2016) Histone deacetylase HDA9 negatively regulates salt and drought stress responsiveness in Arabidopsis. J Exp Bot 67(6):1703–1713
Baek D, Jiang J, Chung JS, Wang B, Chen J, Xin Z, Shi H (2011) Regulated AtHKT1 gene expression by a distal enhancer element and DNA methylation in the promoter plays an important role in salt tolerance. Plant Cell Physiol 52:149–161
Sako K, Kim JM, Matsui A, Nakamura K, Tanaka M, Kobayashi M, Yoshida M (2015) Ky-2, a histone deacetylase inhibitor, enhances high-salinity stress tolerance in Arabidopsis thaliana. Plant Cell Physiol 57(4):776–783
Raju SKK, Shao MR, Wamboldt Y, Mackenzie S (2018) Epigenomic plasticity of Arabidopsis msh1 mutants under prolonged cold stress. bioRxiv 263780
Fu Y, Ma H, Chen S, Gu T, Gong J (2017) Control of proline accumulation under drought via a novel pathway comprising the histone methylase CAU1 and the transcription factor ANAC055. J Exp Bot 69(3):579–588
Huang S, Zhang A, Jin JB, Zhao B, Wang TJ, Wu Y, Wang S, Liu Y, Wang J, Guo P, Ahmad R, Liu B, Xu ZY (2019) Arabidopsis histone H3K4 demethylase JMJ 17 functions in dehydration stress response. New Phytol 223:1372–1387
Arıkan B, Özden S, Turgut-Kara N (2018) DNA methylation related gene expression and morphophysiological response to abiotic stresses in Arabidopsis thaliana. Environ Exp Bot 149:17–26
Fina JP, Casati P (2015) HAG3, a histone acetyltransferase, affects UV-B responses by negatively regulating the expression of DNA repair enzymes and sunscreen content in Arabidopsis thaliana. Plant Cell Physiol 56:1388–1400
Singh P, Yekondi S, Chen P-W, Tsai C-H, Yu C-W, Wu K, Zimmerli L (2014) Environmental history modulates Arabidopsis pattern triggered immunity in a HISTONE ACETYLTRANSFERASE1–dependent manner. Plant Cell 26:2676–2688
Luo M, Wang YY, Liu X, Yang S, Lu Q, Cui Y, Wu K (2012) HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J Exp Bot 6:3297–3306
Buszewicz D, Archacki R, Palusiński A, Kotliński M, Fogtman A, Iwanicka-Nowicka R, Sosnowska K, Kuciński J, Pupel P, Olędzki J, Dadlez M, Misicka A, Jerzmanowski A, Koblowska MK (2016) HD2C histone deacetylase and a SWI/SNF chromatin remodelling complex interact and both are involved in mediating the heat stress response in Arabidopsis. Plant Cell Environ 39:2108–2122
Shi D, Zhuang K, Xia Y, Zhu C, Chen C, Hu Z, Shen Z (2017) Hydrilla verticillata employs two different ways to affect DNA methylation under excess copper stress. Aquat Toxicol 193:97–10
Miryeganeh M, Marlétaz F, Gavriouchkina D, Saze H (2021) De novo genome assembly and in natura epigenomics reveal salinity-induced DNA methylation in the mangrove tree Bruguiera gymnorhiza. New Phytol. https://doi.org/10.1111/nph.17738
Liang D, Zhang Z, Wu H, Huang C, Shuai P, Ye CY, Yin W (2014) Single-base-resolution methylomes of Populus trichocarpa reveal the association between DNA methylation and drought stress. BMC Genet 15(1):S9
Pereira WJ, Pappas M, Grattapaglia D, Pappas G (2020) A cost–effective approach to DNA methylation detection by methyl sensitive DArT sequencing. PLoS ONE 15:e0233800. https://doi.org/10.1371/journal.pone.0233800
Sow MD, Le Gac AL, Fichot R, Lanciano S, Delaunay A, Le-Jan I et al (2021) RNAi suppression of DNA methylation affects the drought stress response and genome integrity in transgenic poplar. New Phytol 2021:17555. https://doi.org/10.1111/nph.17555
Wang MZ, Li HL, Tang M, Yu FH (2022) DNA methylation correlates with responses of experimental Hydrocotyle vulgaris populations to different flood regimes. Front Plant Sci 13:831175. https://doi.org/10.3389/fpls.2022.831175
Sammarco I, Münzbergová Z, Latzel V (2022) DNA methylation can mediate local adaptation and response to climate change in the clonal plant Fragaria vesca: Evidence from a European–scale reciprocal transplant experiment. Front Plant Sci 13:827166. https://doi.org/10.3389/fpls.2022.827166
Lehmair TA, Poschlod P, Reisch C (2022) The impact of environment on genetic and epigenetic variation in Trifolium pratense populations from two contrasting semi–natural grasslands. R Soc Open Sci 9:211406. https://doi.org/10.1098/rsos.211406
Agarwal G, Kudapa H, Ramalingam A, Choudhary D, Sinha P, Garg V, Singh VK, Patil GB, Pandey MK, Nguyen HT et al (2020) Epigenetics and epigenomics: underlying mechanisms, relevance, and implications in crop improvement. Funct Integr Genomics 20:739–761
Wei W, Tao JJ, Chen HW, Li QT, Zhang WK, Ma B, Lin Q, Zhang JS, Chen SY (2017) A histone code reader and a transcriptional activator interact to regulate genes for salt tolerance. Plant Physiol 175:1304–1320
Tsuji H, Saika H, Tsutsumi N, Hirai A, Nakazono M (2006) Dynamic and reversible changes in histone H3-Lys4 methylation and H3 acetylation occurring at submergence-inducible genes in rice. Plant Cell Physiol 47:995–1003
Liu C, Lu F, Cui X, Cao X (2010) Histone methylation in higher plants. Annu Rev Plant Biol 61:395–420
Grativol C, Hemerly AS, Ferreira PCG (2012) Genetic and epigenetic regulation of stress responses in natural plant populations. Biochem Biophys Acta 1819:176–185
Meister G, Tuschl T (2004) Mechanisms of gene silencing by double stranded RNA. Nature 431:343
Maxwell EK, Ryan JF, Schnitzler CE, Browne WE, Baxevanis AD (2012) MicroRNAs and essential components of the microRNA processing machinery are not encoded in the genome of the ctenophore Mnemiopsis leidyi. BMC Genom 13(1):714–723
Xu C, Tian J, Mo B (2013) siRNA-mediated DNA methylation and H3K9 dimethylation in plants. Protein Cell 4(9):656–663
Mosher RA, Schwach F, Studholme D, Baulcombe DC (2008) PolIVb infuences RNA-directed DNA methylation independently of its role in siRNA biogenesis. PNAS 105:3145–3150
Xie M, Yu B (2015) siRNA-directed DNA methylation in plants. Curr Genomics 16(1):23–31
Dalakouras A, Wassenegger M (2013) Revisting RNA-directed DNA methylation. RNA Biol 10(3):453–455
Boyko A, Kovalchuk I (2008) Epigenetic control of plant stress response. Environ Mol Mutagen 49:61–72
Zemach A, McDaniel IE, Silva P, Zilberman D (2010) Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 4:328–338
Choi CS, Sano H (2007) Abiotic-stress induces demethylation and transcriptional activation of a gene encoding a glycerophosphodiesterase-like protein in tobacco plants. Mol Genet Genomics 277(5):589–600
Dyachenko OV, Zakharchenko NS, Shevchuk TV, Bohnert HJ, Buryanov YI (2006) Effect of hypermethylation of CCWGG sequences in DNA of Mesembryanthemum crystallinum plants on their adaptation to salt stress. Biochemistry 71(4):461–465
Kovarik A, Koukalova B, Bezdek M, Opatrny Z (1997) Hypermethylation of tobacco heterochromatic loci in response to osmotic stress. Theor Appl Genet 95:301–306
Zilberman D, Gehring M, Tran RK, Ballinger T, Henikof S (2007) Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers interdependence between methylation and transcription. Natl Genet 39:61–69
Cantu D, Vanzetti LS, Sumner A, Dubcovsky M, Matvienko M, Distelfeld A, Michelmore RW, Dubcovsky J (2010) Small RNAs, DNA methylation and transposable elements in wheat. BMC Genomics 11(1):408
Hashida SN, Uchiyama T, Martin C, Kishima Y, Sano Y, Mikami T (2006) The temperature- dependent change in methylation of the Antirrhinum transposon Tam3 is controlled by the activity of its transposase. Plant Cell 18:104–118
Hirochika H, Sugimoto K, Otsuki Y, Tsugawa H, Kanda M (1996) Retrotransposons of rice involved in mutations induced by tissue culture. Proc Natl Acad Sci USA 93:7783–7788
Beguiristain T, Grandbastien MA, Puigdomenech P, Casacuberta M (2001) Three Tnt1 subfamilies show diferent stress-associated pattern of expression in tobacco. Consequences for retrotransposon control and evolution in plants. Plant Physiol 127:212–222
Kalendar R, Tanskanen J, Immonen S, Nevo E, Schulman AH (2000) Genome evolution of wild barley (Hordeum spontaneum) by BARE-1 retrotransposon dynamics in response to sharp microclimatic divergence. Proc Nat Acad Sci 97(12):6603–6660
Cavrak VV, Lettner N, Jamge S, Kosarewicz A, Bayer LM et al (2014) How a retrotransposon exploits the plant’s heat stress response for its activation. PLoS Genet 10(1):1004115
Chen LT, Luo M, Wang YY, Wu K (2010) Involvement of Arabidopsis histone deacetylase HDA6 in ABA and salt stress response. J Exp Bot 61:3345–3353
Sokol A, Kwiatkowska A, Jerzmanowski A, Prymakowska-Bosak M (2007) Up-regulation of stress- inducible genes in tobacco and Arabidopsis cells in response to abiotic stresses and ABA treatment correlates with dynamic changes in histone H3 and H4 modifcations. Planta 227:245–254
Kim J, To T, Ishida J, Morosawa T, Kawashima M, Matsui A et al (2009) Alterations of lysine modifications on the histone H3 N-tail under drought stress conditions in Arabidopsis thaliana. Plant Cell Physiol 50:1856–1864
Cloix C, Jenkins GI (2008) Interaction of the Arabidopsis UV-B-specific signalling component UVR8 with chromatin. Mol Plant 1:118–128
Boyko A, Golubov A, Bilichak A, Kovalchuk I (2010) Chlorine ions but not sodium ions alter genome stability of Arabidopsis thaliana. Plant Cell Physiol 51(6):1066–1078
Zeller G, Henz SR, Widmer CK, Sachsenberg T, Ratsch G, Weigel D, Laubinger S (2009) Stress- induced changes in the Arabidopsis thaliana transcriptome analyzed using whole-genome tiling arrays. Plant J 58:1068–1082
Yan Y, Zhang Y, Yang K, Sun Z, Fu Y, Chen X et al (2011) Small RNAs from MITE derived stem- loop precursors regulate abscisic acid signalling and abiotic stress responses in rice. Plant J 65:820–828
Borsani O, Zhu J, Verslues PE, Sunkar R, Zhu JK (2005) Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell 123:1279–1291
Schwab R, Maizel A, Ruiz-Ferrer V, Garcia D, Bayer M, Crespi M et al (2009) Endogenous TasiRNAs mediate non-cell autonomous effects on gene regulation in Arabidopsis thaliana. PLoS ONE 4:5980
Lister R, Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, Millar AH, Ecker JR (2008) Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133(3):523–536
Zhong X, Wang ZQ, Xiao R, Wang Y, Xie Y, Zhou X (2017) iTRAQ analysis of the tobacco leaf proteome reveals that RNA-directed DNA methylation (RdDM) has important roles in defense against geminivirus-betasatellite infection. J Proteomics 152:88–101
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16(8):2001–2019
Lv D, Bai X, Li Y, Ding X, Ge Y, Cai H et al (2010) Profiling of cold-stress-responsive miRNAs in rice by microarrays. Gene 459:39–47
Zhang J, Xu Y, Huan Q, Chong K (2009) Deep sequencing of Brachypodium small RNAs at the global genome level identifies micro RNAs involved in cold stress response. BMC Genomics 10:449
Kim DH, Doyle MR, Sung S, Amasino RM (2009) Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol 25:277–299
Whittaker C, Dean C (2017) The FLC locus: a platform for discoveries in epigenetics and adaptation. Annu Rev Cell Dev Biol 33:555–575
Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C (2004) Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427:164–167
Csorba T, Questa JI, Sun Q, Dean C (2014) Antisense COOLAIR mediates the coordinated switching of chromatin states at FLC during vernalization. Proc Natl Acad Sci USA 111:16160–16165
Castaings L, Bergonzi S, Albani MC, Kemi U, Savolainen O, Coupland G (2014) Evolutionary conservation of cold-induced antisense RNAs of FLOWERING LOCUS C in Arabidopsis thaliana perennial relatives. Nat Commun 5:4457
Heo JB, Sung S (2011) Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 331:76–79
Shanker A, Venkateswarlu B, eds (2011) Abiotic stress in plants: mechanisms and adaptations. InTech, Rijeka, p 42
Brzezinka K, Altmann S, Czesnick H, Nicolas P, Gorka M, Benke E, Kabelitz T, Jähne F, Graf A (2016) Arabidopsis FORGETTER1 mediates stress-induced chromatin memory through nucleosome remodeling. eLife 5:e17061
Gallusci P, Dai Z, Génard M, Gauffretau A, Leblanc-Fournier N, Richard-Molard C, Vile D, Brunel- Muguet S (2017) Epigenetics for plant improvement: current knowledge and modeling avenues. Trends Plant Sci 22:610–623
Mozgova I, Mikulski P, Pecinka A, Farrona S (2019) Epigenetic mechanisms of abiotic stress response and memory in plants. In: Alvarez-Ve De-la-Peña C, Casas-Mollano JA (eds) Epigenetics in plants of agronomic importance: fundamentals and applications. Springer International, Cham, pp 1–64
Molinier J, Ries G, Zipfel C, Hohn B (2006) Transgeneration memory of stress in plants. Nature 442:1046–1049
Lang-Mladek C, Popova O, Kiok K, Berlinger M, Rakic B, Aufsatz W, Jonak C, Hauser MT, Luschnig C (2010) Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis. Mol Plant 3:594–602
Kwon CS, Lee D, Choi G, Chung WI (2009) Histone occupancy dependent and -independent removal of H3K27 trimethylation at cold-responsive genes in Arabidopsis. Plant J 60:112–121
Pecinka A, Dinh HQ, Baubec T, Rosa M, Lettner N, Mittelsten Scheid O (2010) Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. Plant Cell 22:3118–3129
Jiang D, Berger F (2017) DNA replication-coupled histone modification maintains Polycomb gene silencing in plants. Science 357(6356):1146–1149
Taudt A, Colome-Tatche M, Johannes F (2016) Genetic sources of population epigenomic variation. Nat Rev Genet 17:319–332
Zhang L et al (2017) A natural tandem array alleviates epigenetic repression of IPA1 and leads to superior yielding rice. Nat Commun 8:14789
Chandler VL (2007) Paramutation: from maize to mice. Cell 128:641–645
Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol 43:179–188
Rhee Y, Sekhon RS, Chopra S, Kaeppler S (2010) Tissue culture-induced novel epialleles of a Myb transcription factor encoded by pericarp color1 in maize. Genetics 186:843–855
Ong-Abdullah M, Ordway JM, Jiang N et al (2015) Loss of Karma transposon methylation underlies the mantled somaclonal variant of oil palm. Nature 525:533–537
Tanurdzic M et al (2008) Epigenomic consequences of immortalized plant cell suspension culture. PLoS Biol 6:2880–2895
Stroud H et al (2013) Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife 2:e00354
Stelpflug SC, Eichten SR, Hermanson PJ, Springer NM, Kaeppler SM (2014) Consistent and heritable alterations of DNA methylation are induced by tissue culture in maize. Genetics 198:209–218
Hollick JB (2017) Paramutation and related phenomena in diverse species. Nat Rev Genet 18:5–23
Greaves IK et al (2016) Twenty-four-nucleotide siRNAs produce heritable trans-chromosomal methylation in F1 Arabidopsis hybrids. Proc Natl Acad Sci USA 113:E6895–E6902
Jordan WT, Schmitz RJ (2016) The shocking consequences of hybrid epigenomes. Genome Biol 17:85
Rigal M et al (2016) Epigenome confrontation triggers immediate reprogramming of DNA methylation and transposon silencing in Arabidopsis thaliana F1 epihybrids. Proc Natl Acad Sci USA 113:E2083–E2092
Wendel JF, Jackson SA, Meyers BC, Wing RA (2016) Evolution of plant genome architecture. Genome Biol 17:37
Lane AK, Niederhuth CE, Ji L, Schmitz RJ (2014) pENCODE: a plant encyclopedia of DNA elements. Annu Rev Genet 48:49–70
Turcotte H, Hooker J, Samanfar B, Parent JS (2022) Can epigenetics guide the production of better adapted cultivars? Agronomy 12:838. https://doi.org/10.3390/agronomy12040838
Guarino F, Cicatelli A, Castiglione S, Agius DR, Orhun GE, Fragkostefanakis S et al (2022) An epigenetic alphabet of crop adaptation to climate change. Front Genet 13:818727. https://doi.org/10.3389/fgene.2022.818727
Xia H, Huang W, Xiong J, Tao T, Zheng X, Wei H et al (2016) Adaptive epigenetic differentiation between upland and lowland rice ecotypes revealed by methylation-sensitive amplified polymorphism. PLoS ONE 11(7):e0157810. https://doi.org/10.1371/journal.pone.0157810
Samantara K, Shiv A, de Sousa LL, Sandhu KS, Priyadarshini P, Mohapatra SR (2021) A comprehensive review on epigenetic mechanisms and application of epigenetic modifications for crop improvement. Environ Exp Bot 188:104479. https://doi.org/10.1016/j.envexpbot.2021.104479
Cong W, Miao Y, Xu L, Zhang Y, Yuan C, Wang J et al (2019) Transgenerational memory of gene expression changes induced by heavy metal stress in rice (Oryza sativa L.). BMC Plant Biol 19:282. https://doi.org/10.1186/s12870-019-1887-7
Ashapkin VV, Kutueva LI, Aleksandrushkina NI, Vanyushin BF (2020) Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int J Mol Sci 21:7457. https://doi.org/10.3390/ijms21207457
Papikian A, Liu W, Gallego-Bartolomé J, Jacobsen SE (2019) Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat Commun 10:729. https://doi.org/10.1038/s41467-019-08736-7
De Melo BP, Lourenço-Tessutti IT, Paixão JFR, Noriega DD, Silva MCM, de Almeida-Engler J, Fontes EPB, Grossi-de-Sa MF (2020) Transcriptional modulation of AREB-1 by CRISPRa improves plant physiological performance under severe water deficit. Sci Rep 10:16231. https://doi.org/10.1038/s41598-020-72464-y
Jogam P, Sandhya D, Alok A, Peddaboina V, Allini VR, Zhang B (2022) A review on CRISPR/Cas-based epigenetic regulation in plants. Int J Biol Macromol 219:1261–1271. https://doi.org/10.1016/j.ijbiomac.2022.08.182
Qi Q, Hu B, Jiang W, Wang Y, Yan J, Ma F, Guan Q, Xu J (2023) Advances in plant epigenome editing research and its application in plants. Int J Mol Sci 24:3442. https://doi.org/10.3390/ijms24043442
Hou Q, Wan X (2021) Epigenome and epitranscriptome: potential resources for crop improvement. Int J Mol Sci 22:12912
Moradpour M, Abdulah SNA (2020) CRISPR/dCas9 platforms in plants: strategies and applications beyond genome editing. Plant Biotechnol J 18:32–44
Bilichak A, Kovalchuk I (2016) Transgenerational response to stress in plants and its application for breeding. J Exp Bot 67:2081–2092
Pandey MK et al (2016) Emerging genomic tools for legume breeding: current status and future prospects. Front Plant Sci 7:455
Hofmeister BT, Lee K, Rohr NA, Hall DW, Schmitz RJ (2017) Stable inheritance of DNA methylation allows creation of epigenotype maps and the study of epiallele inheritance patterns in the absence of genetic variation. Genome Biol 18:155
Kooke R, Johannes F, Wardenaar R, Becker F, Etcheverry M, Colot V, Vreugdenhil D, Keurentjes JJB (2015) Epigenetic basis of morphological variation and phenotypic plasticity in Arabidopsis thaliana. Plant Cell 27:337–348
Manning K, Tör M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38:948–952
Hu Y, Morota G, Rosa GJ, Gianola D (2015) Prediction of plant height in Arabidopsis thaliana using DNA methylation data. Genetics 201:779–793
Zhang Y, Andrews H, Eglitis-Sexton J, Godwin I, Tanurdžić M, Crisp PA (2022) Epigenome guided crop improvement: current progress and future opportunities. Emerg Top Life Sci 6:ETLS20210258. https://doi.org/10.1042/ETLS20210258
Bhogireddy S, Kudapa H, Bajaj P, Garg V, Chitikineni A, Nayak S, Varshney RK (2023) Transcriptome analysis of chickpea during heat stress unveils the signatures of long intergenic non-coding RNAs (lincRNAs) and mRNAs in the heat-QTL region. Crop Des 21:100026. https://doi.org/10.1016/j.cropd.2023.100026
Funding
Source of Funding is not applicable for this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
There are no competing interests the authors would like to declare.
Informed consent
The authors declare that they have no conflict of interest. All the authors read and approved the manuscript.
Ethical approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, M., Rani, K. Epigenomics in stress tolerance of plants under the climate change. Mol Biol Rep 50, 6201–6216 (2023). https://doi.org/10.1007/s11033-023-08539-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08539-6