Abstract
Background
Nerium oleander L. is ethnopharmacologically used for diabetes. Our aim was to investigate the ameliorative effects of ethanolic Nerium flower extract (NFE) in STZ-induced diabetic rats.
Methods
Seven random groups including control group, NFE group (50 mg/kg), diabetic group, glibenclamide group and NFE treated groups (25 mg/kg, 75 mg/kg, and 225 mg/kg) were composed of forty-nine rats. Blood glucose level, glycated hemoglobin (HbA1c), insulin level, liver damage parameters and lipid profile parameters were investigated. Antioxidant defense system enzyme activities and reduced glutathione (GSH) and malondialdehyde (MDA) contents and immunotoxic and neurotoxic parameters were determined in liver tissue. Additionally, the ameliorative effects of NFE were histopathologically examined in liver. mRNA levels of SLC2A2 gene encoding glucose transporter 2 protein were measured by quantitative real time PCR.
Results
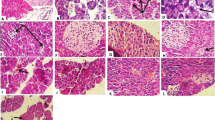
NFE caused decrease in glucose level and HbA1c and increase in insulin and C-peptide levels. Additionally, NFE improved liver damage biomarkers and lipid profile parameters in serum. Moreover, lipid peroxidation was prevented and antioxidant enzyme activities in liver were regulated by NFE treatment. Furthermore, anti-immunotoxic and anti-neurotoxic effects of NFE were determined in liver tissue of diabetic rats. Histopathogically, significant liver damages were observed in the diabetic rats. Histopathological changes were decreased partially in the 225 mg/kg NFE treated group. SLC2A2 gene expression in liver of diabetic rats significantly reduced compared to healthy rats and NFE treatment (25 mg/kg) caused increase in gene expression.
Conclusion
Flower extract of Nerium plant may have an antidiabetic potential due to its high phytochemical content.
Graphical Abstract

Highlights
-
Nerium flower extract (NFE) may have antidiabetic and antioxidant potential.
-
Nerium flower extract (NFE) may have antidiabetic and antioxidant potential.
-
NFE treatment resulted with decrease in lipid peroxidation and liver biomarkers.
-
SLC2A2 gene expression level decreased in liver of diabetic rats.
-
Histopathological improvements were observed in NFE treated diabetic rats.



Similar content being viewed by others
Data Availability
All data generated or analyzed during this study is included in this article.
Ethical Animals were handled according to the guidelines of the Institutional Committee of Care and Use of Laboratory Animals of the Van Yüzüncü Yıl University Experimental Animal Unit, that approved the experiments under the file 27552122-604.01.02-E.41,738.
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WHH, Zhang P, Bommer C, Kuo SH, Boyko EJJ, Magliano DJ et al (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice 183.
Kumar S, Mittal A, Babu D, Mittal A (2021) Herbal Medicines for Diabetes Management and its secondary complications. Curr Diabetes Rev 17(4):437–456
Gould GW, Thomas HM, Jess TJ, Bell GI et al (1991) Expression of human glucose transporters in Xenopus oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry 30(21):5139–5145
Sharari S, Abou-Alloul M, Hussain K, Khan FA et al (2020) Fanconi-Bickel syndrome: a review of the Mechanisms that lead to Dysglycaemia. Int J Mol Sci 21(17):6286
Dolgova AA, Ignateva NS et al (1963) [Morphologo-Anatomical features of Oleander Leaves]. Aptechn Delo 12:36–41
Dey P (2020) The pharmaco-toxicological conundrum of oleander: potential role of gut microbiome. Pharmacotherapy 129:110422
Siddiqui S, Hafeez F, Begum S, Siddiqui BS et al (1986) Isolation and structure of two cardiac glycosides from the leaves of Nerium oleander. Phytochemistry 26(1):237–241
Siddiqui S, Hafeez F, Begum S, Siddiqui BS et al (1988) Oleanderol, a New Pentacyclic Triterpene from the Leaves of Nerium-Oleander. J Nat Prod 51(2):229–233
Siddiqui BS, Begum S, Siddiqui S, Lichter W et al (1995) Two cytotoxic pentacyclic triterpenoids from Nerium oleander. Phytochemistry 39(1):171–174
Begum S, Siddiqui BS, Sultana R, Zia A, Suria A et al (1999) Bio-active cardenolides from the leaves of Nerium oleander. Phytochemistry 50(3):435–438
Fu L, Zhang S, Li N, Wang J, Zhao M, Sakai J, Hasegawa T, Mitsui T, Kataoka T, Oka S, Kiuchi M, Hirose K, Ando M et al (2005) Three new triterpenes from Nerium oleander and biological activity of the isolated compounds. J Nat Prod 68(2):198–206
Wong SK, Lim YY, Ling SK, Chan EWC et al (2014) Caffeoylquinic acids in leaves of selected Apocynaceae species: their isolation and content. Pharmacognosy Res 6(1):67–72
El Sawi NM, Geweely NS, Qusti S, Mohamed M, Kamel A et al (2010) Cytotoxicity and antimicrobial activity of Nerium oleander extracts. J App Anim Res 37(1):25–31
Siddiqui BS, Khatoon N, Begum S, Farooq AD, Qamar K, Bhatti HA, Ali SK et al (2012) Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity. Phytochemistry 77:238–244
Singhal KG, Das Gupta G et al (2012) Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4-induced liver injury in rats. Asian Pac J Trop Med 5(9):677–685
Singh S, Shenoy S, Nehete PN, Yang PY, Nehete B, Fontenot D, Yang GJ, Newman RA, Sastry KJ et al (2013) Nerium oleander derived cardiac glycoside oleandrin is a novel inhibitor of HIV infectivity. Fitoterapia 84:32–39
Ayaz M, Baba F, Akgun N, Bas AL, Uney K, Dik B et al (2015) Protective effect of distillated Nerium oleander on heart of type 2 diabetic rats. Bratisl Lek Listy 116(7):451–456
Balkan IA, Dogan HT, Zengin G, Colak N, Ayaz FA, Goren AC, Kirmizibekmez H, Yesilada E et al (2018) Enzyme inhibitory and antioxidant activities of Nerium oleander L. flower extracts and activity guided isolation of the active components. Ind Crop Prod 112:24–31
Saha MR, Sarker DD, Kar P, Gupta PS, Sen A et al (2014) Indigenous knowledge of plants in local healthcare management practices by tribal people of Malda district, India. J Intercult Ethnopharmacol 3(4):179–185
Mohadjerani M (2012) Antioxidant activity and total phenolic content of Nerium oleander L. grown in North of Iran. Iran J Pharm Res 11(4):1121–1126
Dogan A, Celik I, Kaya MS et al (2015) Antidiabetic properties of lyophilized extract of acorn (Quercus brantii Lindl.) On experimentally STZ-induced diabetic rats. J Ethnopharmacol 176:243–251
Dogan A, Dalar A, Sadullahoglu C, Battal A, Uzun Y, Celik I, Demirel K et al (2018) Investigation of the protective effects of horse mushroom (Agaricus arvensis Schaeff.) Against carbon tetrachloride-induced oxidative stress in rats. Mol Biol Rep 45(5):787–797
Matthews DR, Hosker J, Rudenski A, Naylor B, Treacher D, Turner RJD et al (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocr Metab 85(7):2402–2410
Jain SK, Mcvie R, Duett J, Herbst JJ et al (1989) Erythrocyte-membrane lipid-peroxidation and glycosylated hemoglobin in diabetes. Diabetes 38(12):1539–1543
Uyar A, Yener Z, Dogan A et al (2016) Protective effects of Urtica dioica seed extract in aflatoxicosis: histopathological and biochemical findings. Br Poult Sci 57(2):235–245
Hamza N, Berke B, Umar A, Cheze C, Gin H, Moore N et al (2019) A review of algerian medicinal plants used in the treatment of diabetes. J Ethnopharmacol 238:111841
Dey P, Saha MR, Chowdhuri SR, Sen A, Sarkar MP, Haldar B, Chaudhuri TK et al (2015) Assessment of anti-diabetic activity of an ethnopharmacological plant Nerium oleander through alloxan induced diabetes in mice. J Ethnopharmacol 161:128–137
Gayathri V, Ananthi S, Chandronitha C, Ramakrishnan G, Lakshmisundaram R, Vasanthi HR et al (2011) Cardioprotective effect of Nerium oleander flower against isoproterenol-induced myocardial oxidative stress in experimental rats. J Cardiovasc Pharmacol Ther 16(1):96–104
Singhal KG, Gupta GD et al (2012) Neuroprotective Appraisal of Methanolic Extract of Flowers of Nerium oleander in a non classical rat model of Alzheimer Disease. Nat Prod J 2(3):235–245
Shafiq Y, Naqvi SBS, Rizwani GH, Asghar MA, Bushra R, Ghayas S, Rehman AA, Asghar MA et al (2021) A mechanistic study on the inhibition of bacterial growth and inflammation by Nerium oleander extract with comprehensive in vivo safety profile. BMC Complement Med Ther 21(1):1–19
Okoduwa SIR, Umar IA, James DB, Inuwa HM et al (2017) Appropriate insulin level in selecting fortified diet-fed, streptozotocin-treated rat model of type 2 diabetes for anti-diabetic studies. PLoS ONE 12(1):e0170971
Wickramasinghe ASD, Attanayake AP, Kalansuriya P et al (2022) Biochemical characterization of high fat diet fed and low dose streptozotocin induced diabetic Wistar rat model. J Pharmacol Toxicol Methods 113:107144
Dey P, Saha MR, Roy Choudhuri S, Sarkar I, Halder B, Poddar-Sarkar M, Sen A, Chaudhuri TK et al (2019) Oleander Stem and Root Standardized Extracts Mitigate Acute Hyperglycaemia by Limiting Systemic Oxidative Stress Response in Diabetic Mice. Adv Pharmacol Sci 2019: 7865359
Dogan A, Celik I et al (2016) Healing effects of sumac (Rhus coriaria) in streptozotocin-induced diabetic rats. Pharm Biol 54(10):2092–2102
Agirman E, Celik I, Dogan A et al (2022) Consumption of the syrian mesquite plant (Prosopis farcta) fruit and seed lyophilized extracts may have both protective and toxic effects in STZ-induced diabetic rats. Arch Physiol Biochem 128(4):887–896
Dey P, Dutta S, Biswas-Raha A, Sarkar MP, Chaudhuri TK et al (2016) Haloalkane induced hepatic insult in murine model: amelioration by Oleander through antioxidant and anti-inflammatory activities, an in vitro and in vivo study. BMC Complement Altern Med 16(1):1–15
Rao AA, Sridhar GR, Das UN et al (2007) Elevated butyrylcholinesterase and acetylcholinesterase may predict the development of type 2 diabetes mellitus and Alzheimer’s disease. Med Hypotheses 69(6):1272–1276
Uluoglu C, Cimen B, Ozbey G, Karasu C, Durakoglugil DB, Gunes A, Turkozkan N, Zengil H et al (2008) The effect of experimental diabetes on the circadian pattern of adenosine deaminase and myeloperoxidase activities in rat liver. Gen Physiol Biophys 27(1):25–31
Kurtul N, Pence S, Akarsu E, Kocoglu H, Aksoy Y, Aksoy H et al (2004) Adenosine deaminase activity in the serum of type 2 diabetic patients. Acta Medica (Hradec Kralove) 47(1):33–35
Sacan O, Turkyilmaz IB, Bayrak BB, Mutlu O, Akev N, Yanardag R et al (2021) Protective role of zinc in liver damage in experimental diabetes demonstrated via different biochemical parameters. J Biochem Mol Toxicol 35(1):e22617
Heid CA, Stevens J, Livak KJ, Williams PMJGr et al (1996) Real time quantitative PCR. Genome Res 6(10):986–994
Thorens BJD (2015) GLUT2, glucose sensing and glucose homeostasis. Diabetologia 58(2):221–232
Abdel-Rahman RF, Ezzat SM, Ogaly HA, Abd-Elsalam RM, Hessin AF, Fekry MI, Mansour DF, Mohamed S et al (2020) Ficus deltoidea extract down-regulates protein tyrosine phosphatase 1B expression in a rat model of type 2 diabetes mellitus: a new insight into its antidiabetic mechanism. J Nutr Sci 9(2):1–18
Orci L, Unger RH, Ravazzola M, Ogawa A, Komiya I, Baetens D, Lodish H, Thorens B et al (1990) Reduced beta-cell glucose transporter in new onset diabetic BB rats. J Clin Investig 86(5):1615–1622
Thorens B, Weir GC, Leahy JL, Lodish HF, Bonner-Weir S et al (1990) Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci 87(17):6492–6496
Cao H, Hininger-Favier I, Kelly MA, Benaraba R, Dawson HD, Coves S, Roussel AM, Anderson RA et al (2007) Green tea polyphenol extract regulates the expression of genes involved in glucose uptake and insulin signaling in rats fed a high fructose diet. J Agric Food Chem 55(15):6372–6378
Dey P, Chaudhuri TKJAoBS et al (2016) Comparative phytochemical profiling and effects of Nerium oleander extracts on the activities of murine peritoneal macrophages. 68:515–5313
Atay Balkan İ, Gören AC, Kırmızıbekmez H, Yeşilada E et al (2018) Evaluation of the in vitro anti-inflammatory activity of Nerium oleander L. flower extracts and activity-guided isolation of the active constituents. Rec Nat Prod 12(2):128–141
Alkhalidy H, Moore W, Wang Y, Luo J, McMillan RP, Zhen W, Zhou K, Liu D et al (2018) The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules 23(9):2338
Yang Y, Chen Z, Zhao X, Xie H, Du L, Gao H, Xie C et al (2022) Mechanisms of Kaempferol in the treatment of diabetes: a comprehensive and latest review. Front Endocrinol 13:990299
Ibrahim A, Khalifa SI, Khafagi I, Youssef DT, Khan S, Mesbah M, Khan I et al (2008) Microbial metabolism of biologically active secondary metabolites from Nerium oleander L. Chem Pharm Bull 56(9):1253–1258
Jung SH, Ha YJ, Shim EK, Choi SY, Jin JL, Yun-Choi HS, Lee JR et al (2007) Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem J 403(2):243–250
Acknowledgements
We want to thank to Assoc. Prof. Dr. Süleyman Mesut PINAR because of identification of plant material.
Funding
This work was supported by Van Yüzüncü Yıl University Scientific Research Coordination Unit with grant numbers (TSA-2017-5927) and (TSA-2016-5097).
Author information
Authors and Affiliations
Contributions
Abdulhamit Battal: Development of methodology, analysis, data interpretation, manuscript writing and editing; Abdulahad Dogan: Development of methodology, analysis, data interpretation, manuscript writing and editing; Ahmet Uyar and Ömer Faruk Keleş: Histological studies; Abdulbaki Demir: Analysis data; Ismail Celik: Design of experiment and manuscript review; Mehmet Cengiz Baloglu: Analysis data and manuscript review; Ali Aslan: Data analysis.
Corresponding author
Ethics declarations
Competing Interests
Authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Battal, A., Dogan, A., Uyar, A. et al. Exploring of the ameliorative effects of Nerium (Nerium oleander L.) ethanolic flower extract in streptozotocin induced diabetic rats via biochemical, histological and molecular aspects. Mol Biol Rep 50, 4193–4205 (2023). https://doi.org/10.1007/s11033-023-08332-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08332-5