Abstract
Background
Common treatments of liver disease failed to meet all the needs in this important medical field. It results in an urgent need for proper some new adjuvant therapies. Mesenchymal stem cells (MSCs) and their derivatives are promising tools in this regard. We aimed to compare the Silymarin, as traditional treatment with mesenchymal stem cell conditioned medium (MSC-CM), as a novel strategy, both with therapeutic potentialities in term of liver failure (LF) treatment.
Methods and results
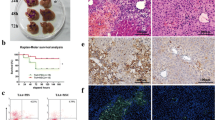
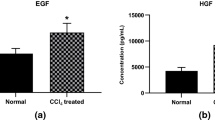
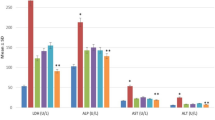
Mice models with liver failure were induced with CCl4 and were treated in the groups as follows: normal mice receiving DMEM-LG medium as control, LF-mice receiving DMEM-LG medium as sham, LF-mice receiving Silymarin as LF-SM, and LF-mice receiving MSC sphere CM as LF-MSC-CM. Biochemical, histopathological, molecular and protein level parameters were evaluated using blood and liver samples. Liver enzymes, MicroRNA-122 values as well as necrotic score were significantly lower in the LF-SM and LF-MSC-CM groups compared to sham. LF-SM showed significantly higher level of total antioxidant capacity and malondialdehyde than that of LF-MSC-CM groups. Sph-MSC-CM not only induced more down-regulated expression of fibrinogen-like protein 1 and receptor interacting protein kinases1 but also led to higher expression level of keratinocyte growth factor. LF-MSC-CM showed less mortality rate compared to other groups.
Conclusions
Hepato-protective potentialities of Sph-MSC-CM are comparable to those of Silymarin. More inhibition of necroptosis/ necrosis and inflammation might result in rapid liver repair in case of MSC-CM administration.





Similar content being viewed by others
References
Kim WR, Brown RS, Terrault NA, El-Serag H (2002) Burden of liver disease in the United States: summary of a workshop. Hepatology 36(1):227–242
Asrani SK, Devarbhavi H, Eaton J, Kamath PS (2019) Burden of liver diseases in the world. J Hepatol 70(1):151–171. https://doi.org/10.1016/j.jhep.2018.09.014
Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L et al (2018) Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 69(3):718–735. https://doi.org/10.1016/j.jhep.2018.05.011
Corey KE, Kaplan LM (2014) Obesity and liver disease: the epidemic of the twenty-first century. Clin Liver Dis 18(1):1–18. https://doi.org/10.1016/j.cld.2013.09.019
Pereira K, Salsamendi J, Casillas J (2015) The global nonalcoholic fatty liver disease epidemic: what a radiologist needs to know. J Clin Imaging Sci. https://doi.org/10.4103/2156-7514.157860
Jin SZ, Liu BR, Xu J, Gao FL, Hu ZJ, Wang XH et al (2012) Ex vivo-expanded bone marrow stem cells home to the liver and ameliorate functional recovery in a mouse model of acute hepatic injury. Hepatobiliary Pancreat Dis Int 11(1):66–73. https://doi.org/10.1016/S1499-3872(11)60127-6
Strom SC, Cai H, Ellis E, Mitamura K, Miki T (2006) Bigger may not be better when it comes to hepatocytes. Liver Transpl 12(1):16–18. https://doi.org/10.1002/lt.20593
Toniutto P, Bitetto D, Fornasiere E, Fumolo E (2019) Challenges and future developments in liver transplantation. Minerva Gastroenterol Dietol 65(2):136–152. https://doi.org/10.23736/S1121-421X.18.02529-1
Stickel F, Schuppan D (2007) Herbal medicine in the treatment of liver diseases. Dig Liver Dis 39(4):293–304. https://doi.org/10.1016/j.dld.2006.11.004
Zhu SY, Jiang N, Jie T, Jing Y, Yue Z (2017) Antioxidant and anti-aging activities of Silybum marianum protein hydrolysate in mice treated with D-galactose. Biomed Environ Sci 30(9):623–631. https://doi.org/10.3967/bes2017.083
Abenavoli L, Izzo AA, Milić N, Cicala C, Santini A, Capasso R (2018) Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res 32(11):2202–2213. https://doi.org/10.1002/ptr.6171
Sahin E, Bagci R, Bektur NE, Kacar S, Sahinturk V (2020) Silymarin attenuated nonalcoholic fatty liver disease through the regulation of endoplasmic reticulum stress proteins GRP78 and XBP-1 in mice. J Food Biochem 44(6):e13194. https://doi.org/10.1111/jfbc.13194
Robert JMS, Peddha MS, Srivastava AK (2021) Effect of Silymarin and Quercetin in a miniaturized scaffold in wistar rats against non-alcoholic fatty liver disease. ACS Omega 6:20735–20745. https://doi.org/10.1021/acsomega.1c00555
Bijak M (2017) Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—Chemistry, bioavailability, and metabolism. Molecules 22(11):1942. https://doi.org/10.3390/molecules22111942
Papackova Z, Heczkova M, Dankova H, Sticova E, Lodererova A, Bartonova L et al (2018) Silymarin prevents acetaminophen-induced hepatotoxicity in mice. PLoS ONE 13(1):e0191353. https://doi.org/10.1371/journal.pone.0191353
Feher J, Lengyel G (2012) Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr Pharm Biotechnol 13(1):210–217. https://doi.org/10.2174/138920112798868818
Ghaneialvar H, Soltani L, Rahmani HR, Lotfi AS, Soleimani M (2018) Characterization and classification of mesenchymal stem cells in several species using surface markers for cell therapy purposes. Ind J Clin Biochem 33(1):46–52. https://doi.org/10.1007/s12291-017-0641-x
Rodini CO, da Silva PBG, Assoni AF, Carvalho VM, Okamoto OK (2018) Mesenchymal stem cells enhance tumorigenic properties of human glioblastoma through independent cell-cell communication mechanisms. Oncotarget 9(37):24766. https://doi.org/10.18632/oncotarget.25346
Friedman SL, Arthur M (1989) Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Investig 84(6):1780–1785. https://doi.org/10.1172/JCI114362
Driscoll J, Patel T (2019) The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J Gastroenterol 5:1–11. https://doi.org/10.1007/s00535-019-01599-1
Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q et al (2018) Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther 9(1):320. https://doi.org/10.1186/s13287-018-1069-9
Tokhanbigli S, Baghaei K, Asadirad A, Hashemi SM, Asadzadeh-Aghdaei H, Zali MR (2019) Immunoregulatory impact of human mesenchymal-conditioned media and mesenchymal derived exosomes on monocytes. Mol Biol Res Commun 3:79–89. https://doi.org/10.22099/mbrc.2019.33346.1397
Sagaradze G, Grigorieva O, Nimiritsky P, Basalova N, Kalinina N, Akopyan Z et al (2019) Conditioned medium from human mesenchymal stromal cells: towards the clinical translation. Int J Mol Sci 20(7):1656. https://doi.org/10.3390/ijms20071656
Rahimi B, Panahi M, Saraygord-Afshari N, Taheri N, Bilici M, Jafari D et al (2021) The secretome of mesenchymal stem cells and oxidative stress: challenges and opportunities in cell-free regenerative medicine. Mol Biol Rep 48:5607–5619. https://doi.org/10.1007/s11033-021-06360-7
Wang YH, Wu DB, Chen B, Chen EQ, Tang H (2018) Progress in mesenchymal stem cell–based therapy for acute liver failure. Stem Cell Res Ther 9(1):227. https://doi.org/10.1186/s13287-018-0972-4
Temnov AA, Rogov KA, Sklifas AN, Klychnikova EV, Hartl M, Djinovic-Carugo K, Charnagalov A (2019) Protective properties of the cultured stem cell proteome studied in an animal model of acetaminophen-induced acute liver failure. Mol Biol Rep 46(3):3101–3112. https://doi.org/10.1007/s11033-019-04765-z
Amiri F, Kiani AA, Bahadori M, Roudkenar MH (2022) Co-culture of mesenchymal stem cell spheres with hematopoietic stem cells under hypoxia: a cost-effective method to maintain self-renewal and homing marker expression. Mol Bio Rep 49(2):931–941. https://doi.org/10.1007/s11033-021-06912-x
Amiri F, Molaei S, Bahadori M, Nasiri F, Deyhim MR, Jalili MA (2016) Autophagy-modulated human bone marrow-derived mesenchymal stem cells accelerate liver restoration in mouse models of acute liver failure. Iran Biomed J 20(3):135. https://doi.org/10.7508/ibj.2016.03.002
Zare H, Jamshidi S, Piryaei A, Dehghan MM, Sasani F, Molaei S et al (2015) Induction of acute hepatic failure by carbon tetrachloride in the nmri mouse model. Qom Univ Med Sci J 9(4):74–84
Ravan AP, Goudarzi F, Rafieemehr H, Bahmani M, Rad F, Jafari M et al (2019) Human umbilical cord-mesenchymal stem cells conditioned medium attenuates CCl4 induced chronic liver fibrosis. Toxin Rev 8:1–12. https://doi.org/10.1080/15569543.2019.1590849
Pawitan JA (2014) Prospect of stem cell conditioned medium in regenerative medicine. BioMed Res Int. https://doi.org/10.1155/2014/965849
McGill MR, Cao M, Svetlov A, Sharpe MR, Williams CD, Curry SC et al (2014) Argininosuccinate synthetase as a plasma biomarker of liver injury after acetaminophen overdose in rodents and humans. Biomarkers 19(3):222–230. https://doi.org/10.3109/1354750X.2014.897757
Kunden RD, Khan JQ, Ghezelbash S, Wilson JA (2020) The role of the liver-specific microRNA, miRNA-122 in the HCV replication cycle. Int J Mol Sci 7(16):5677. https://doi.org/10.3390/ijms21165677
Ning Q, Liu YF, Ye PJ, Gao P, Li ZP, Tang SY et al (2019) Delivery of liver-specific miRNA-122 using a targeted macromolecular prodrug toward synergistic therapy for hepatocellular carcinoma. ACS Appl Mater Interfaces 11(11):10578–10588. https://doi.org/10.1021/acsami.9b00634
Hermenean A, Stan M, Ardelean A, Pilat L, Mihali CV, Popescu C et al (2015) Antioxidant and hepatoprotective activity of milk thistle (Silybum marianum L. Gaertn.) seed oil. Open Life Sci. https://doi.org/10.1515/biol-2015-0017
Parekkadan B, Van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW et al (2007) Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE 2(9):e941. https://doi.org/10.1371/journal.pone.0000941
Khodayar MJ, Kalantari H, Khorsandi L, Ahangar N, Samimi A, Alidadi H (2021) Taurine attenuates valproic acid-induced hepatotoxicity via modulation of RIPK1/RIPK3/MLKL-mediated necroptosis signaling in mice. Mol Biol Rep 48(5):4153–4162. https://doi.org/10.1007/s11033-021-06428-4
Wang L, Chang X, Feng J, Yu J, Chen G (2020) TRADD mediates RIPK1-independent necroptosis induced by tumor necrosis factor. Front Cell Dev Biol 7:393. https://doi.org/10.3389/fcell.2019.00393
Zhang Y, Qiao HX, Zhou YT, Hong L, Chen JH (2018) Fibrinogen-like-protein 1 promotes the invasion and metastasis of gastric cancer and is associated with poor prognosis. Mol Med Rep 18(2):1465–1472. https://doi.org/10.3892/mmr.2018.9097
Xagorari A, Siotou E, Yiangou M, Tsolaki E, Bougiouklis D, Sakkas L et al (2013) Protective effect of mesenchymal stem cell-conditioned medium on hepatic cell apoptosis after acute liver injury. Int J Clin Exp Pathol 6(5):831
Hu C, Zhao L, Duan J, Li L (2019) Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J Cell Mol Med 23(3):1657–1670. https://doi.org/10.1111/jcmm.14115
Bi Z, Zhou Q, Geng Y, Zhang H (2016) Human umbilical cord mesenchymal stem cells ameliorate experimental cirrhosis through activation of keratinocyte growth factor by suppressing microRNA-199. Eur Rev Med Pharmacol Sci 20(23):4905–4912. https://doi.org/10.1155/2021/6685605
Qajari NM, Shafaroudi MM, Gholami M, Khonakdar-Tarsi A (2020) Silibinin treatment results in reducing OPA1&MFN1 genes expression in a rat model hepatic ischemia-reperfusion. Mol Biol Rep 47(5):3271–3280. https://doi.org/10.1007/s11033-020-05383-w
Tvrdý V, Pourová J, Jirkovský E, Křen E, Valentová K, Mladěnka P (2021) Systematic review of pharmacokinetics and potential pharmacokinetic interactions of flavonolignans from silymarin. Med Res Rev 41(4):2195–2246. https://doi.org/10.1002/med.21791
Lotfinia M, Kadivar M, Piryaei A, Pournasr B, Sardari S, Sodeifi N et al (2016) Effect of secreted molecules of human embryonic stem cell-derived mesenchymal stem cells on acute hepatic failure model. Stem Cell Dev 25(24):1898–1908. https://doi.org/10.1089/scd.2016.0244
An SY, Jang YJ, Lim H-J, Han J, Lee J, Lee G et al (2017) Milk fat globule-EGF factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology 152(5):1174–1186. https://doi.org/10.1053/j.gastro.2016.12.003
Huang B, Cheng X, Wang H, Huang W, Wang D, Zhang K et al (2016) Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med 14(1):45. https://doi.org/10.1186/s12967-016-0792-1
Šuk J, Jašprová J, Biedermann D, Petrásková L, Valentová K, Křen V et al (2019) Isolated Silymarin flavonoids increase systemic and hepatic bilirubin concentrations and lower lipoperoxidation in mice. Oxid Med Cell Longev. https://doi.org/10.1155/2019/6026902
Acknowledgements
This work was supported by Hamadan university of medical sciences financially (No: 990202405, Ethic code No: IR.UMSHA.REC.1398.1077). Special thanks to high institute for research and education in transfusion medicine, Tehran, Iran for technical supports.
Funding
This work was supported by Hamadan university of medical sciences financially (No: 990202405).
Author information
Authors and Affiliations
Contributions
Conceptualization: FA, Methodology: SM, SF and MB, Formal analysis: RS, Investigation and Writing—original draft preparation: SM and MB, Review and editing: FA, MB and SF, Funding acquisition: FA, RS, Supervision: FA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: MSCs were isolated from human umbilical cord samples after filling out the consent form by parents.
Research involving human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study was confirmed in ethic committee of Hamadan university of medical sciences (Code No: IR.UMSHA.REC.1398.1077).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Molaei, S., Amiri, F., Salimi, R. et al. Therapeutic effects of mesenchymal stem cells-conditioned medium derived from suspension cultivation or silymarin on liver failure mice. Mol Biol Rep 49, 10315–10325 (2022). https://doi.org/10.1007/s11033-022-07785-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07785-4