Abstract
Background
Targeted knock-in assisted by the CRISPR/Cas9 system is an advanced technology with promising applications in various research fields including medical and agricultural sciences. However, improvements in the efficiency, precision, and specificity of targeted knock-in are prerequisites to facilitate the practical application of this technology. To improve the efficiency of targeted knock-in, it is necessary to have a molecular system that allows sensitive monitoring of targeted knock-in events with simple procedures.
Methods and results
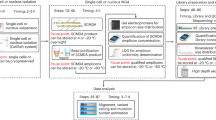
We developed an assay, named CD55 correction assay, with which to monitor CD55 gene correction accomplished by targeted knock-in. To create the reporter clones used in this assay, we initially introduced a 7.7-kb heterozygous deletion covering CD55 exons 2–5, and then incorporated a truncating mutation within exon 4 of the remaining CD55 allele in human cell lines. The resultant reporter clones that lost the CD55 protein on the cell membrane were next transfected with Cas9 constructs along with a donor plasmid carrying wild-type CD55 exon 4. The cells were subsequently stained with fluorescence-labeled CD55 antibody and analyzed by flow cytometry to detect CD55-positive cells. These procedures allow high-throughput, quantitative detection of targeted gene correction events occurring in an endogenous human gene.
Conclusions
The current study demonstrated the utility of the CD55 correction assay to sensitively quantify the efficiency of targeted knock-in. When used with the PIGA correction assay, the CD55 correction assay will help accurately determine the efficiency of targeted knock-in, precluding possible experimental biases caused by cell line-specific and locus-specific factors.



Similar content being viewed by others
Data availability
Data and materials will be available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Anzalone AV, Koblan LW, Liu DR (2020) Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol 38:824–844. https://doi.org/10.1038/s41587-020-0561-9
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821. https://doi.org/10.1126/science.1225829
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N et al (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. https://doi.org/10.1126/science.1231143
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE et al (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826. https://doi.org/10.1126/science.1232033
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V et al (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31:827–832. https://doi.org/10.1038/nbt.2647
Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK et al (2013) High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31:822–826. https://doi.org/10.1038/nbt.2623
Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR (2013) High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol 31:839–843. https://doi.org/10.1038/nbt.2673
Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE et al (2013) Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154:1380–1389. https://doi.org/10.1016/j.cell.2013.08.021
Chen X, Janssen JM, Liu J, Maggio I, T’Jong AEJ, Mikkers HMM et al (2017) In trans paired nicking triggers seamless genome editing without double-stranded DNA cutting. Nat Commun 8:657. https://doi.org/10.1038/s41467-017-00687-1
Nakajima K, Zhou Y, Tomita A, Hirade Y, Gurumurthy CB, Nakada S (2018) Precise and efficient nucleotide substitution near genomic nick via noncanonical homology-directed repair. Genome Res 28:223–230. https://doi.org/10.1101/gr.226027.117
Hyodo T, Rahman ML, Karnan S, Ito T, Toyoda A, Ota A et al (2020) Tandem paired nicking promotes precise genome editing with scarce interference by p53. Cell Rep 30:1195–207.e7. https://doi.org/10.1016/j.celrep.2019.12.064
Karnan S, Konishi Y, Ota A, Takahashi M, Damdindorj L, Hosokawa Y et al (2012) Simple monitoring of gene targeting efficiency in human somatic cell lines using the PIGA gene. PLoS ONE 7:e47389. https://doi.org/10.1371/journal.pone.0047389
Miyata T, Takeda J, Iida Y, Yamada N, Inoue N, Takahashi M et al (1993) The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science 259:1318–1320. https://doi.org/10.1126/science.7680492
Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T et al (1993) Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 73:703–711. https://doi.org/10.1016/0092-8674(93)90250-t
Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R et al (2005) Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 106:3699–3709. https://doi.org/10.1182/blood-2005-04-1717
Brodsky RA (2009) How I treat paroxysmal nocturnal hemoglobinuria. Blood 113:6522–6527. https://doi.org/10.1182/blood-2009-03-195966
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308. https://doi.org/10.1038/nprot.2013.143
Acknowledgements
We would like to thank Mr. Makoto Naruse and colleagues at the Institute of Comprehensive Medical Research, Division of Advanced Research Promotion at Aichi Medical University, for providing technical assistance, and Dr. Feng Zhang (Broad Institute) for providing the plasmids PX458, PX459, and PX462 via Addgene.
Funding
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (JSPS; 18K14703 to TH, 19K09292 to SK, 18K08342 and 21K08426 to AO, 18H02645 to ST, 19K08668 to YH, and 17K07263 and 20K06613 to HK), the Nitto Foundation (to TH), Takeda Science Foundation (to TH and HK, respectively), Hirose International Scholarship Foundation (to SK), Ichihara International scholarship foundation (to HK), and Takahashi Industrial and Economic Research Foundation (to HK). MLR and MNH are supported by the Japanese Government (MEXT) Scholarship for Research Students.
Author information
Authors and Affiliations
Contributions
HK conceived, designed, and supervised the project. HK designed and MLR performed the experiments. TH guided MLR in performing experiments. HK, TH, and MLR analyzed data. MNH, YM, SK, AO, ST, and YH provided insights into the study. HK wrote the manuscript with contribution from MLR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest/competing interests.
Informed consent
Not applicable.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rahman, M.L., Hyodo, T., Hasan, M.N. et al. Correction of a CD55 mutation to quantify the efficiency of targeted knock-in via flow cytometry. Mol Biol Rep 49, 6241–6248 (2022). https://doi.org/10.1007/s11033-022-07422-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07422-0