Abstract
Background
Myeloid cell leukemia-1 (MCL-1) is a component of the Bcl-2 anti-apoptotic family that plays a key role in cell proliferation and differentiation. Despite tremendous improvements toward identification of the role of MCL-1 in leukemia progression, the functional significance and molecular mechanism behind the effect of MCL-1 overexpression on the proliferation of B-cell precursor acute lymphoblastic leukemia (BCP-ALL) has not been clarified. In addition, less well appreciated is the effect of MCL-1 inhibition on the potentiation of doxorubicin-induced apoptosis in BCP-ALL cell lines. In the present study, we aimed to shed light on the anti-cancer properties of S63845, a potent Mcl-1 inhibitor, in BCP-ALL cell lines either alone or in combination with a chemotherapeutic drug.
Methods and results
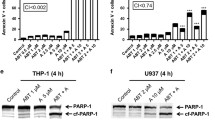
Mononuclear cells from patients with Pre-B ALL and BCP-ALL cell lines were treated with S63845 in presence or absence of doxorubicin, induction of apoptosis was evaluated using Annexin-V/PI staining kit. mRNA and protein expression levels were assessed by qRT-PCR and western blot analysis, respectively. Our results declared that inhibition of Mcl-1 impairs cell growth and induces apoptosis in pre-B ALL cells through activation of caspase-3 and up-regulation of a repertoire of pro-apoptotic Bcl-2 family. Additionally, S63845 acts synergically with doxorubicin to induce apoptosis in BCP-ALL cell lines.
Conclusions
Our data declared that MCL-1 inhibition alone or in combination with a chemotherapeutic agent is considered an appealing strategy for the induction of apoptosis in BCP-ALL cells.





Similar content being viewed by others
Abbreviations
- S:
-
S63845 (Mcl-1 inhibitor)
- Dox:
-
Doxorubicin
- qRT-PCR:
-
Quantitative-real time polymerase chain reaction
References
Aldoss IT, Marcucci G, Pullarkat V (2016) Treatment of acute lymphoblastic leukemia in adults: applying lessons learned in children. Oncology. https://doi.org/10.1007/s12032-009-9347-0
Hunger SP, Mullighan CG (2015) Acute lymphoblastic leukemia in children. New Engl J Med 373:1541–1552. https://doi.org/10.1056/NEJMra1400972
Schroeder MP, Bastian L, Eckert C, Gökbuget N, James AR, Tanchez JO, Schlee C, Isaakidis K, Häupl B, Baum K (2019) Integrated analysis of relapsed B-cell precursor Acute Lymphoblastic Leukemia identifies subtype-specific cytokine and metabolic signatures. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-40786-1
Mullighan CG, the American Society of Hematology Education Program Book (2012) The molecular genetic makeup of acute lymphoblastic leukemia. Hematology 2010 2012:389–396. https://doi.org/10.1182/asheducation.V2012.1.389.3798360
Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:87. https://doi.org/10.1186/1756-9966-30-87
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25:486–541. https://doi.org/10.1038/s41418-017-0012-4
Chen L, Zeng Y, Zhou S-F (2018) Role of apoptosis in cancer resistance to chemotherapy. Curr Underst Apoptosis Progr Cell Death. https://doi.org/10.5772/intechopen.80056
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59. https://doi.org/10.1038/nrm2308
Akl H, Vervloessem T, Kiviluoto S, Bittremieux M, Parys JB, De Smedt H, Bultynck G (2014) A dual role for the anti-apoptotic Bcl-2 protein in cancer: mitochondria versus endoplasmic reticulum. Biochim Biophys Acta (BBA) Mol Cell Res 1843:2240–2252. https://doi.org/10.1016/j.bbamcr.2014.04.017
Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, Chanrion M, Kelly GL, Gong J-N, Moujalled DM (2016) The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538:477–482. https://doi.org/10.1038/nature19830
Heimer S, Knoll G, Schulze-Osthoff K, Ehrenschwender M (2019) Raptinal bypasses BAX, BAK, and BOK for mitochondrial outer membrane permeabilization and intrinsic apoptosis. Cell Death Dis 10:1–13. https://doi.org/10.1038/s41419-019-1790-z
Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M (2010) The landscape of somatic copy-number alteration across human cancers. Nature 463:899–905. https://doi.org/10.1038/nature08822
Wuilleme-Toumi S, Robillard N, Gomez P, Moreau P, Le Gouill S, Avet-Loiseau H, Harousseau J, Amiot M, Bataille R (2005) Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 19:1248–1252. https://doi.org/10.1038/sj.leu.2403784
Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin E, Cidado J, Embrey KJ, Gangl E, Gibbons FD (2018) Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Comm 9:1–14. https://doi.org/10.1038/s41467-018-07551-w
Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, Izon DJ, Zuber J, Rappaport AR, Herold MJ (2012) Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Gene Dev 26:120–125. https://doi.org/10.1101/gad.182980.111
Aichberger KJ, Mayerhofer M, Krauth M-T, Skvara H, Florian S, Sonneck K, Akgul C, Derdak S, Pickl WF, Wacheck V (2005) Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood 105:3303–3311. https://doi.org/10.1182/blood-2004-02-0749
Bolomsky A, Vogler M, Köse MC, Heckman CA, Ehx G, Ludwig H, Caers J (2020) MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J Hematol Oncol 13:1–19. https://doi.org/10.1186/s13045-020-01007-9
Koss B, Morrison J, Perciavalle RM, Singh H, Rehg JE, Williams RT, Opferman JT (2013) Requirement for antiapoptotic MCL-1 in the survival of BCR-ABL B-lineage acute lymphoblastic leukemia. Blood 122:1587–1598. https://doi.org/10.1182/blood-2012-06-440230
Kang MH, Wan Z, Kang YH, Sposto R, Reynolds CP (2008) Mechanism of synergy of N-(4-hydroxyphenyl) retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Canc Inst 100:580–595. https://doi.org/10.1093/jnci/djn076
Wang H, Guo M, Wei H, Chen Y (2021) Targeting MCL-1 in cancer: current status and perspectives. J Hematol Oncol 14:1–18. https://doi.org/10.1186/s13045-021-01079-1
Fletcher S (2019) MCL-1 inhibitors–where are we now (2019)? Expert Opin Ther Pat 29:909–919. https://doi.org/10.1080/13543776.2019.1672661
Alikarami F, Safa M, Faranoush M, Hayat P, Kazemi A (2017) Inhibition of DNA-PK enhances chemosensitivity of B-cell precursor acute lymphoblastic leukemia cells to doxorubicin. Biomed Pharmacother 94:1077–1093. https://doi.org/10.1016/j.biopha.2017.08.022
Sheikh-Zeineddini N, Bashash D, Safaroghli‐Azar A, Riyahi N, Shabestari RM, Janzamin E, Safa M (2019) Suppression of c‐Myc using 10058‐F4 exerts caspase‐3‐dependent apoptosis and intensifies the antileukemic effect of vincristine in pre‐B acute lymphoblastic leukemia cells. J Cell Biochem 120:14004–14016. https://doi.org/10.1002/jcb.28675
Shabestari RM, Safa M, Alikarami F, Banan M, Kazemi A (2017) CREB knockdown inhibits growth and induces apoptosis in human pre-B acute lymphoblastic leukemia cells through inhibition of prosurvival signals. Biomed Pharmacother 87:274–279. https://doi.org/10.1016/j.biopha.2016.12.070
Chou T-C (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70:440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Li Z, He S, Look AT (2019) The MCL1-specific inhibitor S63845 acts synergistically with venetoclax/ABT-199 to induce apoptosis in T-cell acute lymphoblastic leukemia cells. Leukemia 33:262–266. https://doi.org/10.1038/s41375-018-0201-2
Brenner D, Mak TW (2009) Mitochondrial cell death effectors. Curr Opin Cell Biol 21:871–877. https://doi.org/10.1016/j.ceb.2009.09.004
Bhutani M, Kumar L, Vora A, Bhardwaj N, Pathak AK, Singh R, Kochupillai V (2002) Randomized study comparing 4′-epi‐doxorubicin (Epirubicin) versus doxorubicin as a part of induction treatment in adult acute lymphoblastic leukemia. Am J Hematol 71:241–247. https://doi.org/10.1002/ajh.10211
Pugazhendhi A, Edison TNJI, Velmurugan BK, Jacob JA, Karuppusamy I (2018) Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci 200:26–30. https://doi.org/10.1016/j.lfs.2018.03.023
Grabow S, Kelly G, Delbridge A, Kelly P, Bouillet P, Adams J, Strasser A (2016) Critical B-lymphoid cell intrinsic role of endogenous MCL-1 in c-MYC-induced lymphomagenesis. Cell Death Dis 7:e2132–e2132. https://doi.org/10.1038/cddis.2016.43
Fernald K, Kurokawa M (2013) Evading apoptosis in cancer. Trends Cell Biol 23:620–633. https://doi.org/10.1016/j.tcb.2013.07.006
Quinn BA, Dash R, Azab B, Sarkar S, Das SK, Kumar S, Oyesanya RA, Dasgupta S, Dent P, Grant S (2011) Targeting Mcl-1 for the therapy of cancer. Expet Opin Investig Drugs 20:1397–1411. https://doi.org/10.1517/13543784.2011.609167
Merino D, Whittle JR, Vaillant F, Serrano A, Gong J-N, Giner G, Maragno AL, Chanrion M, Schneider E, Pal B (2017) Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci Transl Med 9:eaam7049. https://doi.org/10.1126/scitranslmed.aam7049
Pan R, Ruvolo VR, Wei J, Konopleva M, Reed JC, Pellecchia M, Andreeff M, Ruvolo PP (2015) Inhibition of Mcl-1 with the pan–Bcl-2 family inhibitor (–) BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood 126:363–372. https://doi.org/10.1182/blood-2014-10-604975
Powell JA, Lewis AC, Pitson SM (2017) The MCL-1 inhibitor S63845: an exciting new addition to the armoury of anti-cancer agents. J Xiangya Med 2:1–4
Carr C, Ng J, Wigmore T (2008) The side effects of chemotherapeutic agents. Curr Anaesth Crit Care 19:70–79. https://doi.org/10.1016/j.cacc.2008.01.004
Banerjee S, Li Y, Wang Z, Sarkar FH (2008) Multi-targeted therapy of cancer by genistein. Canc Lett 269:226–242. https://doi.org/10.1016/j.canlet.2008.03.052
Thijssen R, Alvarez-Diaz S, Grace C, Gao M-y, Segal DH, Xu Z, Strasser A, Huang DC (2020) Loss of RIPK3 does not impact MYC-driven lymphomagenesis or chemotherapeutic drug-induced killing of malignant lymphoma cells. Cell Death Differ 27:2531. https://doi.org/10.1038/s41418-020-0576-2
Acknowledgements
This study was derived from the thesis of E Ebrahimi and supported by a grant from Iran University of Medical Science.
Funding
This work was supported by the Grant No. 32662 from Iran University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
MS conceived and designed the research. EEB and RMS conducted the experiment. MS and DB contributed reagent or analytical tools. MS, RMS and DB analyzed data. EEB and RMS wrote the manuscript. MS, DB and RMS revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Elham Ebrahimi, Rima Manafi Shabestari, Davood Bashash, and Majid Safa declares that they have no conflict of interest.
Ethical approval
The procedure performed in present study involving human participants was in accordance with the ethical standards of the Medical Ethic Committee of Iran university of medical science and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all patients and healthy donors included in the study. All authors involved in this study are agree to publish the manuscript in your worthy journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11033_2021_7021_MOESM1_ESM.tif
Supplementary Fig. 1 (a) NALM-6 and (b) SUP-B15 cells were treated with elevating concentrations of S63845 for 24 and 48 hr and cell viability was evaluated with trypan blue method. Dose-effect curve of diverse doses of S63845 in (c) NALM-6 and (d) SUP-B15 cells was measured using MTT assay and expressed using compusyn software. Supplementary material 1 (TIF 302.5 kb)
11033_2021_7021_MOESM2_ESM.tif
Supplementary Fig. 2 The apoptotic cell graphs for NALM-6 (a) and SUP-B15 cells (b) assessed by flowcytometery. Following the treatment of (c) NALM-6 and (d) SUP-B15 cells with mentioned doses of S63845 for 24 hr, caspase-3 activity assay was performed using a colorimetric caspase-3 kit (n=3, *P<0.05, **P<0.01, compared to control cells). Supplementary material 2 (TIF 395.6 kb)
11033_2021_7021_MOESM3_ESM.tif
Supplementary Fig. 3 (a) NALM-6 and (b) SUP-B15 cells were treated with diverse concentrations of doxorubicin and metabolic activity of cells was measured using MTT assay. The Dose-effect curve of different concentrations of Doxorubicin in was depicted using compusyn software. Supplementary material 3 (TIF 151.8 kb)
11033_2021_7021_MOESM4_ESM.tif
Supplementary Fig. 4 (a) NALM-6 cells were incubated with 500 nM concentrations of S63845 in the presence or absence of 200 nM doxorubicin for 24 hr. (b) SUP-B15 cells were cultured in medium containing 60 nM S63845 either with or without 20 nM doxorubicin for 24 hr. Total RNA was extracted and cDNA was prepared. BAX and BCL-2 mRNA expression levels were measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and normalized to the expression of ACTIN (n=3, *P<0.05, **P<0.01 compared to Dox treated cells). Supplementary material 4 (TIF 181.2 kb)
11033_2021_7021_MOESM5_ESM.tif
Supplementary Fig. 5 (a) NALM-6 cells and (b) SUP-B15 cells were treated with indicated doses of MCL-1 S63845 and doxorubicin for 24 hr. Following extraction of total RNA and cDNA preparation, gene expression of C-MYC, hTERT, and MCL-1 was measured by qRT-PCR. All values were normalized to ACTIN. (n=3; *p<0.05, compared to cells treated with doxorubicin alone). Supplementary material 5 (TIF 296.1 kb)
11033_2021_7021_MOESM6_ESM.tif
Supplementary Fig. 6 Mononuclear cells from healthy donors (a) and patients with BCP-ALL (b) were treated with doxorubicin in present or absence of S63845 for 24hr. (*p<0.05, compared to cells treated with doxorubicin alone). Supplementary material 6 (TIF 1394.3 kb)
Rights and permissions
About this article
Cite this article
Ebrahimi, E., Shabestari, R.M., Bashash, D. et al. Synergistic apoptotic effect of Mcl-1 inhibition and doxorubicin on B-cell precursor acute lymphoblastic leukemia cells. Mol Biol Rep 49, 2025–2036 (2022). https://doi.org/10.1007/s11033-021-07021-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-07021-5