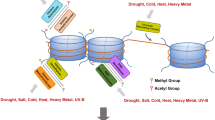
Abstract
Cereals are important crops and are exposed to various types of environmental stresses that affect the overall growth and yield. Among the various abiotic stresses, salt stress is a major environmental factor that influences the genetic, physiological, and biochemical responses of cereal crops. Epigenetic regulation which includes DNA methylation, histone modification, and chromatin remodelling plays an important role in salt stress tolerance. Recent studies in rice genomics have highlighted that the epigenetic changes are heritable and therefore can be considered as molecular signatures. An epigenetic mechanism under salinity induces phenotypic responses involving modulations in gene expression. Association between histone modification and altered DNA methylation patterns and differential gene expression has been evidenced for salt sensitivity in rice and other cereal crops. In addition, epigenetics also creates stress memory that helps the plant to better combat future stress exposure. In the present review, we have discussed epigenetic influences in stress tolerance, adaptation, and evolution processes. Understanding the epigenetic regulation of salinity could help for designing salt-tolerant varieties leading to improved crop productivity.


Similar content being viewed by others
References
Shewry PR, Sandra JH (2015) The contribution of wheat to human diet and health. Food Energy Secur 4(3):178–202. https://doi.org/10.1002/fes3.64
Panta S, Flowers T, Lane P, Doyle R, Haros G, Shabala S (2014) Halophyte agriculture: success stories. Environ Exp Bot 107:71–83. https://doi.org/10.1016/j.envexpbot.2014.05.006
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1787. https://doi.org/10.3389/fpls.2016.01787
Zörb C, Geilfus CM, Dietz KJ (2019) Salinity and crop yield. Plant Biol 21:31–38. https://doi.org/10.1111/plb.12884
Adem GD, Roy SJ, Zhou M, Bowman JP, Shabala S (2014) Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol 14(1):1–13. https://doi.org/10.1186/1471-2229-14-113
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Soda N, Sharan A, Gupta BK, Singla-Pareek SL, Pareek A (2016) Evidence for nuclear interaction of a cytoskeleton protein (OsIFL) with metallothionein and its role in salinity stress tolerance. Sci Rep 6:34762. https://doi.org/10.1038/srep34762
Kumar S, Beena AS, Awana M, Singh A (2017) Physiological, biochemical, epigenetic and molecular analyses of wheat (Triticumaestivum) genotypes with contrasting salt tolerance. Front Plant Sci 8:1151. https://doi.org/10.3389/fpls.2017.01151
Zhu H, Wang G, Qian J (2016) Transcription factors as readers and effectors of DNA methylation. Nat Rev Genet 17(9):551–565. https://doi.org/10.1038/nrg.2016.83
Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK et al (2001) AtHKT1 is a salt tolerance determinant that controls Na entry into plant roots. Proc Natl Acad Sci USA 98:14150–14155. https://doi.org/10.1073/pnas.241501798
Suprasanna P (2020) Plant abiotic stress tolerance: insights into resilience build-up. J Biosci 45:120. https://doi.org/10.1007/s12038-020-00088-5
Iwasaki M, Paszkowski J (2014) Epigenetic memory in plants. EMBO J 33(18):1987–1998. https://doi.org/10.15252/embj.201488883
Thiebaut F, Hemerly AS, Ferreira PCG (2019) A role for epigenetic regulation in the adaptation and stress responses of non-model plants. Front Plant Sci 10:246. https://doi.org/10.3389/fpls.2019.00246
Friedrich T, Faivre L, Bäurle I, Schubert D (2019) Chromatin-based mechanisms of temperature memory in plants. Plant Cell Environ 42:762–770. https://doi.org/10.1111/pce.13373
Duan CG, Zhu JK, Cao X (2018) Retrospective and perspective of plant epigenetics in China. J Genet Genomics 45(11):621–638. https://doi.org/10.1016/j.jgg.2018.09.004
Pikaard CS, Scheid OM (2014) Epigenetic regulation in plants. CSH Perspect Biol 6(12):a019315. https://doi.org/10.1101/cshperspect.a019315
Duan H, Li J, Zhu Y, Jia W, Wang H, Jiang L, Zhou Y (2020) Responsive changes of DNA methylation in wheat (Triticum aestivum) under water deficit. Sci Rep 10(1):1–8. https://doi.org/10.1038/s41598-020-64660-7
Makarevitch I, Waters AJ, West PT, Stitzer M, Hirsch CN, Ibarra JR et al (2015) Transposable elements contribute to activation of maize genes in response to abiotic stress. PLoS Genet 11:e1005566. https://doi.org/10.1371/journal.pgen.1004915
Paul A, Dasgupta P, Roy D, Chaudhuri S (2017) Comparative analysis of Histone modifications and DNA methylation at OsBZ8 locus under salinity stress in IR64 and Nonabokra rice varieties. Plant Mol Biol 95(1):63–88. https://doi.org/10.1007/s11103-017-0636-2
Pandey G, Yadav CB, Sahu PP, Muthamilarasan M, Prasad M (2017) Salinity induced differential methylation patterns in contrasting cultivars of foxtail millet (Setariaitalica L.). Plant Cell Rep 36(5):759–772. https://doi.org/10.1007/s00299-016-2093-9
Wang W, Zhao X, Pan Y, Zhu L, Fu B, Li Z (2011) DNA methylation changes detected by methylation-sensitive amplified polymorphism in two contrasting rice genotypes under salt stress. J Genet Genomics 38(9):419–424. https://doi.org/10.1016/j.jgg.2011.07.006
Zhong L, Xu YH, Wang JB (2009) DNA-methylation changes induced by salt stress in wheat Triticum aestivum. Afr J Biotech 8:6201–6207. https://doi.org/10.5897/AJB09.1058
Zhu N, Cheng S, Liu X, Du H, Dai M, Zhou DX, Yang W, Zhao Y (2015) The R2R3-type MYB gene OsMYB91 has a function in coordinating plant growth and salt stress tolerance in rice. Plant Sci 236:146–156. https://doi.org/10.1016/j.plantsci.2015.03.023
Karan R, DeLeon T, Biradar H, Subudhi PK (2012) Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PLoS ONE 7(6):e40203. https://doi.org/10.1371/journal.pone.0040203
Ferreira LJ, Donoghue MT, Barros P, Saibo NJ, Santos AP, Oliveira MM (2019) Uncovering differentially methylated regions (DMRs) in a salt-tolerant rice variety under stress: one step towards new regulatory regions for enhanced salt tolerance. Epigenomes 3(1):4. https://doi.org/10.3390/epigenomes3010004
Garg R, Chevala VN, Shankar R, Jain M (2015) Divergent DNA methylation patterns associated with gene expression in rice cultivars with contrasting drought and salinity stress response. Sci Rep 5(1):1–16. https://doi.org/10.1038/srep14922
Wang W, Huang F, Qin Q et al (2015) Comparative analysis of DNA methylation changes in two rice genotypes under salt stress and subsequent recovery. Biochem Biophys Res Commun 465:790–796. https://doi.org/10.1016/j.bbrc.2015.08.089
Bocchini M, D’Amato R, Ciancaleoni S, Fontanella MC, Palmerini CA, Beone GM et al (2018) Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic responses to water stress in Zea mays L. by inducing a higher drought tolerance. Front Plant Sci 9:389
Migicovsky Z, Yao Y, Kovalchuk I (2014) Transgenerational phenotypic and epigenetic changes in response to heat stress in Arabidopsis thaliana. Plant Signal Behav 9(2):e27971. https://doi.org/10.4161/psb.27971
Cong W, Miao Y, Xu L, Zhang Y, Yuan C, Wang J et al (2019) Transgenerational memory of gene expression changes induced by heavy metal stress in rice (Oryzasativa L.). BMC Plant Biol 19(1):1–14. https://doi.org/10.1186/s12870-019-1887-7
Dezar CA, Giacomelli JI, Manavella PA, Ré DA, Alves-Ferreira M, Baldwin IT et al (2011) HAHB10, a sunflower HD-Zip II transcription factor, participates in the induction of flowering and in the control of phytohormone-mediated responses to biotic stress. J Exp Bot 62(3):1061–1076. https://doi.org/10.1093/jxb/erq339
Zheng Y, Ding Y, Sun X et al (2016) Histone deacetylase HDA9 negatively regulates salt and drought stress responsiveness in Arabidopsis. J Exp Bot 67:1703–1713. https://doi.org/10.1093/jxb/erv562
Liu J, Zhi P, Wang X, Fan Q, Chang C (2019) Wheat WD40-repeat protein TaHOS15 functions in a histone deacetylase complex to fine-tune defense responses to Blumeriagraminis f. sp. tritici. J Exp Bot 70(1):255–268. https://doi.org/10.1093/jxb/ery330
Liu JG, Han X, Yang T, Cui WH, Wu AM, Fu CX et al (2019) Genome-wide transcriptional adaptation to salt stress in populus. BMC Plant Biol 19(1):1–14. https://doi.org/10.1186/s12870-019-1952-2
Cicatelli A, Todeschini V, Lingua G, Biondi S, Torrigiani P, Castiglione S (2014) Epigenetic control of heavy metal stress response in mycorrhizal versus non-mycorrhizal poplar plants. Env Sci Pollut Res 21(3):1723–1737. https://doi.org/10.1007/s11356-013-2072-4
Neves DM, da Hora Almeida LA, Santana-Vieira DDS, Freschi L, Ferreira CF, dos Santos SoaresFilho W et al (2017) Recurrent water deficit causes epigenetic and hormonal changes in citrus plants. Sci Rep 7(1):1–11. https://doi.org/10.1038/s41598-017-14161-x
González RM, Ricardi MM, Iusem ND (2013) Epigenetic marks in an adaptive water stress-responsive gene in tomato roots under normal and drought conditions. Epigenetics 8(8):864–872. https://doi.org/10.4161/epi.25524
Fan SK, Ye JY, Zhang LL, Chen HS, Zhang HH, Zhu YX et al (2020) Inhibition of DNA demethylation enhances plant tolerance to cadmium toxicity by improving iron nutrition. Plant Cell Env 43(1):275–291. https://doi.org/10.1111/pce.13670
Bharti P, Mahajan M, Vishwakarma AK, Bhardwaj J, Yadav SK (2015) AtROS1 overexpression provides evidence for epigenetic regulation of genes encoding enzymes of flavonoid biosynthesis and antioxidant pathways during salt stress in transgenic tobacco. J Exp Bot 66(19):5959–5969
Verkest A, Byzova M, Martens C, Willems P, Verwulgen T, Slabbinck B et al (2015) Selection for improved energy use efficiency and drought tolerance in canola results in distinct transcriptome and epigenome changes. Plant Physiol 168(4):1338–1350. https://doi.org/10.1104/pp.15.00155
Zhang H, Lang Z, Zhu JK (2018) Dynamics and function of DNA methylation in plants. Nat Rev Mol Cell Biol 19(8):489–506. https://doi.org/10.1038/s41580-018-0016-z
Kim JH (2019) Chromatin remodeling and epigenetic regulation in plant DNA damage repair. Int J Mol Sci 20(17):4093. https://doi.org/10.3390/ijms20174093
Vergara Z, Gutierrez C (2017) Emerging roles of chromatin in the maintenance of genome organization and function in plants. Genome Biol 18(1):1–12. https://doi.org/10.1186/s13059-017-1236-9
Simon SA, Meyers BC (2011) Small RNA-mediated epigenetic modifications in plants. Curr Opin Plant Biol 14(2):148–155. https://doi.org/10.1016/j.pbi.2010.11.007
He XJ, Chen T, Zhu JK (2011) Regulation and function of DNA methylation in plants and animals. Cell Res 21(3):442–465. https://doi.org/10.1038/cr.2011.23
Wu SC, Zhang Y (2010) Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 11(9):607–620. https://doi.org/10.1038/nrm2950
Zhang H, Zhu JK (2012) Active DNA demethylation in plants and animals. CSH Symp Quant Biol 77:161–173. https://doi.org/10.1101/sqb.2012.77.014936
Liu S, Dunwell TL, Pfeifer GP, Dunwell JM, Ullah I, Wang Y (2013) Detection of oxidation products of 5-methyl-2′-deoxycytidine in Arabidopsis DNA. PLoS ONE 8(12):e84620. https://doi.org/10.1371/journal.pone.0084620
Zhou C, Wang C, Liu H, Zhou Q, Liu Q, Guo Y, Peng T, Song J, Zhang J, Chen L, Zhao Y (2018) Identification and analysis of adenine N 6-methylation sites in the rice genome. Nat Plants 4(8):554–563. https://doi.org/10.1038/s41477-018-0214-x
Matzke MA, Mosher RA (2014) RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat Rev Genet 15(6):394–408. https://doi.org/10.1038/nrg3683
Watanabe T, Tomizawa SI, Mitsuya K, Totoki Y, Yamamoto Y, Kuramochi-Miyagawa S, Iida N, Hoki Y, Murphy PJ, Toyoda A, Gotoh K (2011) Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse Rasgrf1 locus. Science 332(6031):848–852. https://doi.org/10.1126/science.1203919
Karanthamalai J, Chodon A, Chauhan S, Pandi G (2020) DNA N6-Methyladenine modification in plant genomes—a glimpse into emerging epigenetic code. Plants 9:247. https://doi.org/10.3390/plants9020247
Halter T, Wang J, Amesefe D, Lastrucci E, Charvin M, Rastogi MS, Navarro L (2021) The Arabidopsis active demethylase ROS1 cis-regulates defence genes by erasing DNA methylation at promoter-regulatory regions. Elife 10:e62994. https://doi.org/10.7554/eLife.62994.sa2
Villagómez-Aranda AL, García-Ortega LF, Torres-Pacheco I, Guevara-González RG (2021) Whole-genome DNA methylation analysis in hydrogen peroxide overproducing transgenic tobacco resistant to biotic and abiotic stresses. Plants 10(1):178. https://doi.org/10.3390/plants10010178
Xin C, Chi J, Zhao Y, He Y, Guo J (2019) Cadmium stress alters cytosine methylation status and expression of a select set of genes in Nicotiana benthamiana. Plant Sci 284:16–24. https://doi.org/10.1016/j.plantsci.2019.03.021
Zhou M, Sng NJ, Le Frois C, Paul AL, Ferl RJ (2019) Epigenomics in an extraterrestrial environment: organ-specific alteration of DNA methylation and gene expression elicited by spaceflight in Arabidopsis thaliana. BMC Genom 20:205. https://doi.org/10.1186/s12864-019-5554-z
Wibowo A, Becker C, Marconi G, Durr J, Price J, Hagmann J, Papareddy R, Putra H, Kageyama J, Weigel D (2016) Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. Elife 5:e13546. https://doi.org/10.7554/eLife.13546.001
Liu J, He Z (2020) Small DNA methylation, big player in plant abiotic stress responses and memory. Front Plant Sci 1011:595603. https://doi.org/10.3389/fpls.2020.595603
Ma Y, Min L, Wang M, Wang C, Zhao Y, Li Y et al (2018) Disrupted genome methylation in response to high temperature has distinct affects on microspore abortion and anther indehiscence. Plant Cell 30:1387–1403. https://doi.org/10.1105/tpc.18.00074
Guo H, Wu T, Li S, He Q, Yang Z, Zhang W, Gan Y, Sun P, Xiang G, Zhang H, Deng H (2019) The methylation patterns and transcriptional responses to chilling stress at the seedling stage in rice. Int J Mol Sci 20(20):5089. https://doi.org/10.3390/ijms20205089
Min L, Li Y, Hu Q, Zhu L, Gao W, Wu Y et al (2014) Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol 164:1293–1308. https://doi.org/10.1104/pp.113.232314
Chachar S, Liu J, Zhang P, Riaz A, Guan C, Liu S (2021) Harnessing current knowledge of DNA N6-methyladenosine from model plants for non-model crops. Front Genet 12:668317. https://doi.org/10.3389/fgene.2021.668317
Chen Q, Chen X, Wang Q, Zhang F, Lou Z, Zhang Q, Zhou DX (2013) Structural basis of a histone H3 lysine 4 demethylase required for stem elongation in rice. PLoS Genet 9(1):e1003239. https://doi.org/10.1371/journal.pgen.1003239
Cui X, Jin P, Cui X, Gu L, Lu Z, Xue Y, Wei L, Qi J, Song X, Luo M, An G, Cao X (2013) Control of transposon activity by a histone H3K4 demethylase in rice. Proc Natl Acad Sci USA 110(5):1953–1958. https://doi.org/10.1073/pnas.1217020110
Hou Y, Wang L, Wang L, Liu L, Li L, Sun L, Rao Q, Zhang J, Huang S (2015) JMJ704 positively regulates rice defense response against Xanthomonasoryzae pv. oryzae infection via reducing H3K4me2/3 associated with negative disease resistance regulators. BMC Plant Biol 15(1):286. https://doi.org/10.1186/s12870-015-0674-3
Liu K, Yu Y, Dong A, Shen WH (2017) SET DOMAIN GROUP701 encodes a H3K4-methytransferase and regulates multiple key processes of rice plant development. New Phytol 215(2):609–623. https://doi.org/10.1111/nph.14596
Yokoo T, Saito H, Yoshitake Y, Xu Q, Asami T, Tsukiyama T, Teraishi M, Okumoto Y, Tanisaka T (2014) Se14, encoding a JmjC domain-containing protein, plays key roles in long-day suppression of rice flowering through the demethylation of H3K4me3 of RFT1. PLoS ONE 9(4):e96064. https://doi.org/10.1371/journal.pone.0096064
Kong L, Liu Y, Wang X, Chang C (2020) Insight into the role of epigenetic processes in abiotic and biotic stress response in wheat and barley. Int J Mol Sci 21(4):1480. https://doi.org/10.3390/ijms21041480
Wang M, Qin L, Xie C, Li W, Yuan J, Kong L, Yu W, Xia G, Liu S (2014) Induced and constitutive DNA methylation in a salinity-tolerant wheat introgression line. Plant Cell Physiol 55(7):1354–1365. https://doi.org/10.1093/pcp/pcu059
Rajkumar MS, Shankar R, Garg R, Jain M (2019) Role of DNA methylation dynamics in desiccation and salinity stress responses in rice cultivars. BioRxiv. https://doi.org/10.1101/558064
Harris CJ, Scheibe M, Wongpalee SP, Liu W, Cornett EM et al (2018) A DNA methylation reader complex that enhances gene transcription. Science 362(6419):1182–1186. https://doi.org/10.1126/science.aar7854
Xiao X, Zhang J, Li T, Fu X, Satheesh V, Niu Q et al (2019) A group of SUVH methyl-DNA binding proteins regulate expression of the DNA demethylase ROS1 in Arabidopsis. J Integr Plant Biol 61(2):110–119. https://doi.org/10.1111/jipb.12768
Singroha G, Sharma P (2019) Epigenetic modifications in plants under abiotic stress. In: Meccariello R (ed) Epigenetics. Intechopen, London
Bartels A, Han Q, Nair P, Stacey L, Gaynier H, Mosley M, Huang QQ, Pearson JK, Hsieh TF, An YQC, Xiao W (2018) Dynamic DNA methylation in plant growth and development. Int J Mol Sci 19(7):2144. https://doi.org/10.3390/ijms19072144
Shen Y, Conde e Silva N, Audonnet L, Servet C, Wei W, Zhou DX (2014) Over-expression of histone H3K4 demethylase gene JMJ15 enhances salt tolerance in Arabidopsis. Front Plant Sci 5:290. https://doi.org/10.3389/fpls.2014.00290
Ganguly DR, Crisp PA, Eichten SR, Pogson BJ (2017) The Arabidopsis DNA methylome is stable under transgenerational drought stress. Plant Physiol 175(4):1893–1912. https://doi.org/10.1104/pp.17.00744
Bräutigam K, Cronk Q (2018) DNA methylation and the evolution of developmental complexity in plants. Front Plant Sci 9:1447. https://doi.org/10.3389/fpls.2018.01447
Allis CD, Jenuwein T (2016) The molecular hallmarks of epigenetic control. Nat Rev Genet 17(8):487–500. https://doi.org/10.1038/nrg.2016.59
Chang YN, Zhu C, Jiang J, Zhang H, Zhu JK, Duan CG (2020) Epigenetic regulation in plant abiotic stress responses. J Integr Plant Biol 62(5):563–580. https://doi.org/10.1111/jipb.12901
Eichten SR, Schmitz RJ, Springer NM (2014) Epigenetics: beyond chromatin modifications and complex genetic regulation. Plant Physiol 165(3):933–947. https://doi.org/10.1104/pp.113.234211
Kouzarides T (2007) Chromatin modifications and their function. Cell 128(4):693–705. https://doi.org/10.1016/j.cell.2007.02.005
Asensi-Fabado MA, Amtmann A, Perrella G (1860) Plant responses to abiotic stress: the chromatin context of transcriptional regulation. Biochim Biophys Acta Gene Regul Mech 1:106–122. https://doi.org/10.1016/j.bbagrm.2016.07.015
Luo M, Cheng K, Xu Y, Yang S, Wu K (2017) Plant responses to abiotic stress regulated by histone deacetylases. Front Plant Sci 8:2147. https://doi.org/10.3389/fpls.2017.02147
Ueda M, Seki M (2020) Histone modifications form epigenetic regulatory networks to regulate abiotic stress response. Plant Physiol 182(1):15. https://doi.org/10.1104/pp.19.00988
Xu Y, Zhang S, Lin S, Guo Y, Deng W, Zhang Y, Xue Y (2017) WERAM: a database of writers, erasers and readers of histone acetylation and methylation in eukaryotes. Nucleic Acids Res 45(D1):D264–D270. https://doi.org/10.1093/nar/gkw1011
Cheng X, Zhang S, Tao W, Zhang X, Liu J, Sun J, Zhang H, Pu L, Huang R, Chen T (2018) INDETERMINATE SPIKELET1 recruits histone deacetylase and a transcriptional repression complex to regulate rice salt tolerance. Plant Physiol 178(2):824–837. https://doi.org/10.1104/pp.18.00324
Lee HG, Seo PJ (2019) MYB96 recruits the HDA15 protein to suppress negative regulators of ABA signaling in Arabidopsis. Nat Commun 10(1):1–14. https://doi.org/10.1038/s41467-019-09417-1
Ma S, Tang N, Li X, Xie Y, Xiang D, Fu J, Shen J, Yang J, Tu H, Li X, Hu H (2019) Reversible histone H2B monoubiquitination fine-tunes abscisic acid signaling and drought response in rice. Mol Plant 12(2):263–277. https://doi.org/10.1016/j.molp.2018.12.005
Hu Z, Song N, Zheng M et al (2015) Histone acetyltransferase GCN5 is essential for heat stress-responsive gene activation and thermotolerance in Arabidopsis. Plant J 84:1178–1191. https://doi.org/10.1111/tpj.13076
Zheng M, Liu X, Lin J, Liu X, Wang Z, Xin M, Yao Y, Peng H, Zhou DX, Ni Z, Sun Q, Hu Z (2019) Histone acetyltransferase GCN 5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J 97(3):587–602. https://doi.org/10.1111/tpj.14144
Bilichak A, Ilnystkyy Y, Hollunder J, Kovalchuk I (2012) The progeny of Arabidopsis thaliana plants exposed to salt exhibit changes in DNA methylation, histone modifications and gene expression. PLoS ONE 7(1):e30515. https://doi.org/10.1371/journal.pone.0030515
Sharma C, Kumar S, Saripalli G, Jain N, Raghuvanshi S, Sharma JB, Prabhu KV, Sharma PK, Gupta PK (2019) H3K4/K9 acetylation and Lr28-mediated expression of six leaf rust responsive genes in wheat (Triticum aestivum). Mol Genet Genom 294(1):227–241. https://doi.org/10.1007/s00438-018-1500-z
Eom SH, Hyun TK (2018) Histone acetyltransferases (HATs) in Chinese cabbage: insights from histone H3 acetylation and expression profiling of HATs in response to abiotic stresses. J Am Soc Hortic Sci 143:296–303. https://doi.org/10.21273/JASHS04436-18
Li H, Yan S, Zhao L et al (2014) Histone acetylation associated up-regulation of the cell wall related genes is involved in salt stress induced maize root swelling. BMC Plant Biol 14:1–14. https://doi.org/10.1186/1471-2229-14-105
Wang T, Xing J, Liu Z et al (2019) Histone acetyltransferase GCN5-mediated regulation of long non-coding RNA At4 contributes to phosphate starvation response in Arabidopsis. J Exp Bot 70:6337–6348. https://doi.org/10.1093/jxb/erz359
Fang H, Liu X, Thorn G et al (2014) Expression analysis of histone acetyltransferases in rice under drought stress. Biochem Biophys Res Commun 443:400–405. https://doi.org/10.1016/j.bbrc.2013.11.102
Zhao J, Zhang J, Zhang W, Wu K, Zheng F, Tian L, Liu X, Duan J (2015) Expression and functional analysis of the plant-specific histone deacetylase HDT701 in rice. Front Plant Sci 5:764. https://doi.org/10.3389/fpls.2014.00764
Ojolo SP, Cao S, Priyadarshani SVGN, Li W, Yan M, Aslam M, Zhao H, Qin Y (2018) Regulation of plant growth and development: a review from a chromatin remodeling perspective. Front Plant Sci 9:1232. https://doi.org/10.3389/fpls.2018.01232
Raxwal VK, Ghosh S, Singh S, Agarwal SK, Goel S, Jagannath A et al (2020) Abiotic stress-mediated modulation of the chromatin landscape in Arabidopsis thaliana. J Exp Bot 71:5280–5293. https://doi.org/10.1093/jxb/eraa286
Han SK, Wu MF, Cui S, Wagner D (2015) Roles and activities of chromatin remodeling ATPases in plants. Plant J 83(1):62–77. https://doi.org/10.1111/tpj.12877
Clapier CR, Cairns BR (2009) The biology of chromatin remodeling complexes. Annu Rev Biochem 78:273–304. https://doi.org/10.1146/annurev.biochem.77.062706.153223
Hargreaves DC, Crabtree GR (2011) ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res 21(3):396–420. https://doi.org/10.1038/cr.2011.32
Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128(4):707–719. https://doi.org/10.1016/j.cell.2007.01.015
Peterson CL, Dingwall A, Scott MP (1994) Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement. Proc Natl Acad Sci USA 91(8):2905–2908. https://doi.org/10.1073/pnas.91.8.2905
Sang Y, Silva-Ortega CO, Wu S, Yamaguchi N, Wu MF, Pfluger J, Gillmor CS, Gallahar KL, Wagner D (2012) Mutations in two non-canonical Arabidopsis SWI2/SNF2 chromatin remodeling ATPases cause embryogenesis and stem cell maintenance defects. Plant J 72(6):1000–1014. https://doi.org/10.1111/tpj.12009
Corona DF, Tamkun JW (2004) Multiple roles for ISWI in transcription, chromosome organization and DNA replication. Biochim Biophys Acta Gene Struc Expres 1677(1–3):113–119. https://doi.org/10.1016/j.bbaexp.2003.09.018
Ebbert R, Birkmann A, Schüller HJ (1999) The product of the SNF2/SWI2 paralogue INO80 of Saccharomyces cerevisiae required for efficient expression of various yeast structural genes is part of a high-molecular-weight protein complex. Mol Microbiol 32(4):741–751. https://doi.org/10.1046/j.1365-2958.1999.01390.x
Berriri S, Gangappa SN, Kumar SV (2016) SWR1 chromatin-remodeling complex subunits and H2A. Z have non-overlapping functions in immunity and gene regulation in Arabidopsis. Mol Plant 9(7):1051–1065. https://doi.org/10.1016/j.molp.2016.04.003
Fritsch O, Benvenuto G, Bowler C, Molinier J, Hohn B (2004) The INO80 protein controls homologous recombination in Arabidopsis thaliana. Mol Cell 16(3):479–485. https://doi.org/10.1016/j.molcel.2004.09.034
Brzezinka K, Altmann S, Czesnick H, Nicolas P, Gorka M, Benke E, Kabelitz T, Jähne F, Graf A, Kappel C, Bäurle I (2016) Arabidopsis FORGETTER1 mediates stress-induced chromatin memory through nucleosome remodeling. Elife 5:e17061. https://doi.org/10.7554/eLife.17061
Song ZT, Liu JX, Han JJ (2021) Chromatin remodeling factors regulate environmental stress responses in plants. J Integr Plant Biol 63(3):438–450. https://doi.org/10.1111/jipb.13064
Han SK, Sang Y, Rodrigues A, Wu MF, Rodriguez PL, Wagner D, Biol F (2012) The SWI2/SNF2 chromatin remodeling ATPase BRAHMA represses abscisic acid responses in the absence of the stress stimulus in Arabidopsis. Plant Cell 24:4892–4906. https://doi.org/10.1105/tpc.112.105114
Peirats-Llobet M, Han SK, Gonzalez-Guzman M, Jeong CW, Rodriguez L, Belda-Palazon B, Wagner D, Rodriguez PL (2015) A direct link between abscisic acid sensing and the chromatin-remodeling ATPase BRAHMA via core ABA signaling pathway components. Mol Plant 9:136–147. https://doi.org/10.1016/j.molp.2015.10.003
Yu X, Meng X, Liu Y, Wang X, Wang TJ, Zhang A, Li N, Qi X, Liu B, Xu ZY (2019) The chromatin remodeler ZmCHB101 impacts alternative splicing contexts in response to osmotic stress. Plant Cell Rep 38:131–145. https://doi.org/10.1007/s00299-018-2354-x
Kim DH, Rossi JJ (2008) RNAi mechanisms and applications. Biotechniques 44(5):613–616. https://doi.org/10.2144/000112792
Duempelmann L, Skribbe M, Bühler M (2020) Small RNAs in the transgenerational inheritance of epigenetic information. Trends Genet 36(3):203–214. https://doi.org/10.1016/j.tig.2019.12.001
Axtell MJ, Westholm JO, Lai EC (2011) Vive la différence: biogenesis and evolution of microRNAs in plants and animals. Genome Biol 12(4):1–13. https://doi.org/10.1186/gb-2011-12-4-221
Chapman EJ, Carrington JC (2007) Specialization and evolution of endogenous small RNA pathways. Nat Rev Genet 8(11):884–896. https://doi.org/10.1038/nrg2179
Shabalina SA, Koonin EV (2008) Origins and evolution of eukaryotic RNA interference. Trends Ecol Evol 23(10):578–587. https://doi.org/10.1016/j.tree.2008.06.005
Czech B, Hannon GJ (2011) Small RNA sorting: matchmaking for Argonautes. Nat Rev Genet 12(1):19–31. https://doi.org/10.1038/nrg2916
Joshua-Tor L, Hannon GJ (2011) Ancestral roles of small RNAs: an ago-centric perspective. CSH Perspect Biol 3(10):a003772. https://doi.org/10.1101/cshperspect.a003772
Khraiwesh B, Zhu JK, Zhu J (1819) Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta Gene Regul Mech 2:137–148. https://doi.org/10.1016/j.bbagrm.2011.05.001
Sunkar R, Chinnusamy V, Zhu J, Zhu JK (2007) Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci 12(7):301–309. https://doi.org/10.1016/j.tplants.2007.05.001
Xie M, Yu B (2015) siRNA-directed DNA methylation in plants. Curr Genom 16(1):23–31. https://doi.org/10.2174/1389202915666141128002211
Sudan J, Raina M, Singh R (2018) Plant epigenetic mechanisms: role in abiotic stress and their generational heritability. 3 Biotech 8(3):172. https://doi.org/10.1007/s13205-018-1202-6
Bologna NG, Voinnet O (2014) The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev Plant Biol 65:473–503. https://doi.org/10.1146/annurev-arplant-050213-035728
Cagirici HB, Alptekin B, Budak H (2017) RNA sequencing and co-expressed long non-coding RNA in modern and wild wheats. Sci Rep 7(1):1–16. https://doi.org/10.1038/s41598-017-11170-8
Huang Y, Li L, Smith KP, Muehlbauer GJ (2016) Differential transcriptomic responses to Fusarium graminearum infection in two barley quantitative trait loci associated with Fusarium head blight resistance. BMC Genomics 17(1):387. https://doi.org/10.1186/s12864-016-2716-0
Shumayla SS, Taneja M, Tyagi S, Singh K, Upadhyay SK (2017) Survey of high throughput RNA-Seq data reveals potential roles for lncRNAs during development and stress response in bread wheat. Front Plant Sci 8:1019. https://doi.org/10.3389/fpls.2017.01019
Unver T, Tombuloglu H (2020) Barley long non-coding RNAs (lncRNA) responsive to excess boron. Genomics 112(2):1947–1955. https://doi.org/10.1016/j.ygeno.2019.11.007
Zhang H, Chen X, Wang C, Xu Z, Wang Y, Liu X, Kang Z, Ji W (2013) Long non-coding genes implicated in response to stripe rust pathogen stress in wheat (Triticumaestivum L.). Mol Biol Rep 40(11):6245–6253. https://doi.org/10.1007/s11033-013-2736-7
Banerjee A, Roychoudhury A (2018) The gymnastics of epigenomics in rice. Plant Cell Rep 37(1):25–49. https://doi.org/10.1007/s00299-017-2192-2
Axtell MJ (2013) Classification and comparison of small RNAs from plants. Annu Rev Plant Biol 64:137–159. https://doi.org/10.1146/annurev-arplant-050312-120043
Wu L, Zhou H, Zhang Q, Zhang J, Ni F, Liu C, Qi Y (2010) DNA methylation mediated by a microRNA pathway. Mol Cell 38(3):465–475. https://doi.org/10.1016/j.molcel.2010.03.008
Hong H, Liu Y, Zhang H, Xiao J, Li X, Wang S (2015) Small RNAs and gene network in a durable disease resistance gene-mediated defense responses in rice. PLoS ONE 10(9):e0137360. https://doi.org/10.1371/journal.pone.0137360
Jung I, Ahn H, Shin SJ, Kim J, Kwon HB, Jung W, Kim S (2016) Clustering and evolutionary analysis of small RNAs identify regulatory siRNA clusters induced under drought stress in rice. BMC Syst Biol 10(4):423–432. https://doi.org/10.1186/s12918-016-0355-3
Wei L, Gu L, Song X, Cui X, Lu Z, Zhou M, Wang L, Hu F, Zhai J, Meyers BC, Cao X (2014) Dicer-like 3 produces transposable element-associated 24-nt siRNAs that control agricultural traits in rice. Proc Natl Acad Sci USA 111(10):3877–3882. https://doi.org/10.1073/pnas.1318131111
Yan Y, Zhang Y, Yang K, Sun Z, Fu Y, Chen X, Fang R (2011) Small RNAs from MITE-derived stem-loop precursors regulate abscisic acid signaling and abiotic stress responses in rice. Plant J 65(5):820–828. https://doi.org/10.1111/j.1365-313x.2010.04467.x
Yang M, Xu Z, Zhao W, Liu Q, Li Q, Lu L, Liu R, Zhang X, Cui F (2018) Rice stripe virus-derived siRNAs play different regulatory roles in rice and in the insect vector Laodelphax striatellus. BMC Plant Biol 18(1):219. https://doi.org/10.1186/s12870-018-1438-7
Fan Y, Yang J, Mathioni SM, Yu J, Shen J, Yang X, Wang L, Zhang Q, Cai Z, Xu C, Li X (2016) PMS1T, producing phased small-interfering RNAs, regulates photoperiod-sensitive male sterility in rice. Proc Natl Acad Sci USA 113(52):15144–15149. https://doi.org/10.1073/pnas.1619159114
Niu D, Zhang X, Song X, Wang Z, Li Y, Qiao L, Wang Z, Liu J, Deng Y, He Z, Yang D (2018) Deep sequencing uncovers rice long siRNAs and its involvement in immunity against Rhizoctonia solani. Phytopathol 108(1):60–69. https://doi.org/10.1094/phyto-03-17-0119-r
Liu X, Li D, Zhang D, Yin D, Zhao Y, Ji C, Zhao X, Li X, He Q, Chen R, Hu S (2018) A novel antisense long noncoding RNA, TWISTED LEAF, maintains leaf blade flattening by regulating its associated sense R2R3-MYB gene in rice. New Phytol 218(2):774–788. https://doi.org/10.1111/nph.15023
Lu Y, Zhou DX, Zhao Y (2020) Understanding epigenomics based on the rice model. Theoret Appl Genet 133(5):1–19. https://doi.org/10.1007/s00122-019-03518-7
Author information
Authors and Affiliations
Contributions
MMR, AV, and RKV wrote the manuscript draft. PS, PS, and RKG critically revised the article. The manuscript has been read and approved by all the authors before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
In compliance with the ethical standards, this article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rashid, .M., Vaishnav, A., Verma, R.K. et al. Epigenetic regulation of salinity stress responses in cereals. Mol Biol Rep 49, 761–772 (2022). https://doi.org/10.1007/s11033-021-06922-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06922-9