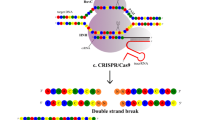
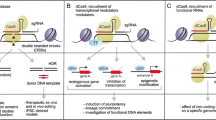
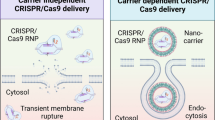
Abstract
Regenerative medicine, a therapeutic approach using stem cells, aims to rejuvenate and restore the normalized function of the cells, tissues, and organs that are injured, malfunctioning, and afflicted. This influential technology reaches its zenith when it is integrated with the CRISPR-Cas (clustered regularly interspaced short palindromic repeats—CRISPR associated) technology of genome editing. This tool acts as a programmable restriction enzyme system, which targets DNA as well as RNA and gets redeployed for the customization of DNA/RNA sequences. The dynamic behaviour of nuclear manipulation and transcriptional regulation by CRISPR-Cas technology renders it with numerous employment in the field of biologics and research. Here, the possible impact of the commonly practiced CRISPR-Cas systems in regenerative medicines is being reviewed. Primarily, the discussion of the working mechanism of this system and the fate of stem cells will be scrutinized. A detailed description of the CRISPR based regenerative therapeutic approaches for a horde of diseases like genetic disorders, neural diseases, and blood-related diseases is elucidated.
Graphic abstract


Similar content being viewed by others
Data availability
Please contact author for data requests.
Abbreviations
- CRISPR-Cas:
-
Clustered repeat interspaced short palindromic repeats/CRISPR associated proteins
- iPSC:
-
Induced pluripotent stem cells
- TALEN:
-
Transcription activator-like endonucleases
- ZFN:
-
Zinc finger nucleases
- RNP:
-
Ribonucleotide protein
- PAM:
-
Protospacer adjacent motiff
- crRNA:
-
CRISPR RNA
- NHEJ:
-
Non homologous end joining
- HDR:
-
Homology-directed repair
- tracrRNA:
-
Trans activating crRNA
- dsRNA:
-
Double stranded RNA
- BMP4:
-
Bone morphogenic protein 4
- UM-MSC:
-
Umbilical cord-derived mesenchymal stem cells
- OM:
-
Osteogenic differentiation media
- CRISPRai:
-
CRISPR activation/repression
- BMSC:
-
Bone marrow-derived mesenchymal stem cells
- GDNF:
-
Glial cell line-derived growth factor
- BDNF:
-
Brain-derived neurotrophic factor
- NGF:
-
Nerve growth factor
- ALS:
-
Amyotrophic lateral sclerosis
- sALS:
-
Sporadic amyotrophic lateral sclerosis
- fALS:
-
Familial amyotrophic lateral sclerosis
- SOD1:
-
Cu–Zn superoxide dismutase 1
- TDP-43:
-
TAR DNA binding protein 43
- VCP:
-
Valosin containing protein
- ANG:
-
Angiogenin
- OPTN:
-
Optineurin
- HLC:
-
Hepatocyte-like cells
- RDEB:
-
Recessive dystrophic epidermolysisbullosa
- DMD:
-
Duchenne muscular dystrophy
- OA:
-
Osteoarthritis
- PH1:
-
Primary hyperoxaluria type 1
- CFTR:
-
Cystic fibrosis transmembrane regulator
8. References
Badylak SF, Nerem RM (2010) Progress in tissue engineering and regenerative medicine. Proc Natl Acad Sci USA 107(8):3285–3286. https://doi.org/10.1073/pnas.1000256107
Wobma H, Vunjak-Novakovic G (2016) Tissue engineering and regenerative medicine 2015: a year in review. Tissue Eng B: Rev 22(2):101–113. https://doi.org/10.1089/ten.TEB.2015.0535
Park KM, Shin YM, Kim K, Shin H (2018) Tissue engineering and regenerative medicine 2017: a year in review. Tissue Eng B: Rev 24(5):327–344. https://doi.org/10.1089/ten.teb.2017.0081
Ding Q, Regan SN, Xia Y, Oostrom LA, Cowan CA, Musunuru K (2013) Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell 12(4):393. https://doi.org/10.1016/j.stem.2013.03.006
Hendriks WT, Jiang X, Daheron L, Cowan CA (2015) TALEN-and CRISPR/Cas9-mediated gene editing in human pluripotent stem cells using lipid-based transfection. Curr Protoc Stem Cell Biol. https://doi.org/10.1016/j.stem.2015.12.002
Lei Y, Lee CL, Joo KI, Zarzar J, Liu Y, Dai B et al (2011) Gene editing of human embryonic stem cells via an engineered baculoviral vector carrying zinc-finger nucleases. Mol Ther 19(5):942–950. https://doi.org/10.1038/mt.2011.12
Chen KY, Knoepfler PS (2016) To CRISPR and beyond: the evolution of genome editing in stem cells. Regener Med 11(8):801–816. https://doi.org/10.2217/rme-2016-0107
Zhang Z, Zhang Y, Gao F, Han S, Cheah KS, Tse HF, Lian Q (2017) CRISPR/Cas9 genome-editing system in human stem cells: current status and future prospects. Mol Ther-Nucl Acids 9:230–241. https://doi.org/10.1016/j.omtn.2017.09.009
Garate Z, Davis BR, Quintana-Bustamante O, Segovia JC (2013) New frontier in regenerative medicine: site-specific gene correction in patient-specific induced pluripotent stem cells. Hum Gene Ther 24(6):571–583. https://doi.org/10.1089/hum.2012.251
Smith C, Ye Z, Cheng L (2016) Genome editing in human pluripotent stem cells. Cold Spring Harbor Protoc. https://doi.org/10.1101/pdb.top086819
Sharma DK (2016) New technologies in regenerative medicine. Indian J Genet Mol Res 5(1):19
Svendsen CN (2013) Back to the future: how human induced pluripotent stem cells will transform regenerative medicine. Hum Mol Genet 22(R1):R32–R38. https://doi.org/10.1093/hmg/ddt379
Koonin EV, Makarova KS, Zhang F (2017) Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol 37:67–78. https://doi.org/10.1016/j.mib.2017.05.008
Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P et al (2011) Evolution and classification of the CRISPR–Cas systems. Nat Rev Microbiol 9(6):467–477. https://doi.org/10.1038/nrmicro2577
Amitai G, Sorek R (2016) CRISPR–Cas adaptation: insights into the mechanism of action. Nat Rev Microbiol 14(2):67. https://doi.org/10.1038/nrmicro.2015.14
Van Der Oost J, Westra ER, Jackson RN, Wiedenheft B (2014) Unravelling the structural and mechanistic basis of CRISPR–Cas systems. Nat Rev Microbiol 12(7):479–492. https://doi.org/10.1038/nrmicro3279
Hille F, Charpentier E (2016) CRISPR-Cas: biology, mechanisms and relevance. Philos Trans R Soc B 371(1707):20150496. https://doi.org/10.1098/rstb.2015.0496
Rath D, Amlinger L, Rath A, Lundgren M (2015) The CRISPR-Cas immune system: biology, mechanisms and applications. Biochimie 117:119–128. https://doi.org/10.1016/j.biochi.2015.03.025
Makarova KS, Aravind L, Wolf YI, Koonin EV (2011) Unification of Cas protein families and a simple scenario for the origin and evolution of CRISPR-Cas systems. Biol Direct 6(1):1–27. https://doi.org/10.1186/1745-6150-6-38
Wang J, Zhang C, Feng B (2020) The rapidly advancing Class 2 CRISPR-Cas technologies: a customizable toolbox for molecular manipulations. J Cell Mol Med 24(6):3256–3270. https://doi.org/10.1111/jcmm.15039
Wilbie D, Walther J, Mastrobattista E (2019) Delivery aspects of CRISPR/Cas for in vivo genome editing. Acc Chem Res 52(6):1555–1564. https://doi.org/10.1021/acs.accounts.9b00106
Wang D, Mou H, Li S, Li Y, Hough S, Tran K et al (2015) Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum Gene Ther 26(7):432–442. https://doi.org/10.1089/hum.2015.087
Naso MF, Tomkowicz B, Perry WL, Strohl WR (2017) Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 31(4):317–334. https://doi.org/10.1007/s40259-017-0234-5
Thummel R, Bai S, SarrasJr MP, Song P, McDermott J, Brewer J et al (2006) Inhibition of zebrafish fin regeneration using in vivo electroporation of morpholinos against fgfr1 and msxb. Dev Dyn 235(2):336–346. https://doi.org/10.1002/dvdy.20630
Horii T, Arai Y, Yamazaki M, Morita S, Kimura M, Itoh M et al (2014) Validation of microinjection methods for generating knockout mice by CRISPR/Cas-mediated genome engineering. Sci Rep 4(1):1–6. https://doi.org/10.1038/srep04513
Glass Z, Lee M, Li Y, Xu Q (2018) Engineering the delivery system for CRISPR-based genome editing. Trends Biotechnol 36(2):173–185. https://doi.org/10.1016/j.tibtech.2017.11.006
Wang M, Zuris JA, Meng F, Rees H, Sun S, Deng P et al (2016) Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc Natl Acad Sci USA 113(11):2868–2873. https://doi.org/10.1073/pnas.1520244113
Tong R, Christian DA, Tang L, Cabral H, Baker JR, Kataoka K et al (2009) Nanopolymeric therapeutics. MRS Bull 34(6):422–431. https://doi.org/10.1557/mrs2009.118
Ramakrishna S, Dad ABK, Beloor J, Gopalappa R, Lee SK, Kim H (2014) Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res 24(6):1020–1027. https://doi.org/10.1101/gr.171264.113
Mout R, Ray M, Yesilbag Tonga G, Lee YW, Tay T, Sasaki K, Rotello VM (2017) Direct cytosolic delivery of CRISPR/Cas9-ribonucleoprotein for efficient gene editing. ACS Nano 11(3):2452–2458. https://doi.org/10.1021/acsnano.6b07600
Bongso A, Lee EH (2005) Stem cells: their definition, classification and sources. Stem Cells. https://doi.org/10.1142/9789812569370_0001
Ilic D, Polak JM (2011) Stem cells in regenerative medicine: introduction. Br Med Bull 98(1):117–126. https://doi.org/10.1093/bmb/ldr012
Hipp J, Atala A (2008) Sources of stem cells for regenerative medicine. Stem Cell Rev 4(1):3–11. https://doi.org/10.1007/s12015-008-9010-8
Keller GM (1995) In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 7(6):862–869. https://doi.org/10.1016/0955-0674(95)80071-9
Götherström C, Ringden O, Westgren M, Tammik C, Le Blanc K (2003) Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Trans 32(3):265–272. https://doi.org/10.1038/sj.bmt.1704111
Ringe J, Kaps C, Burmester GR, Sittinger M (2002) Stem cells for regenerative medicine: advances in the engineering of tissues and organs. Naturwissenschaften 89(8):338–351. https://doi.org/10.1007/s00114-002-0344-9
Verfaillie CM (2002) Adult stem cells: assessing the case for pluripotency. Trends Cell Biol 12(11):502–508. https://doi.org/10.1016/s0962-8924(02)02386-3
Yang J, Yamato M, Nishida K, Ohki T, Sekine M, Sekine H et al (2006) Cell delivery in regenerative medicine: the cell sheet engineering approach. J Control Release 116(2):193–203. https://doi.org/10.1016/j.jconrel.2006.06.022
Choi J, Bae T, Byambasuren N, Park SH, Jo CH, Kim D et al (2020) CRISPR-Cpf1 activation of endogenous BMP4 gene for osteogenic differentiation of umbilical-cord-derived mesenchymal stem cells. Molr Ther-Methods Clin Dev 17:309–316. https://doi.org/10.1016/j.omtm.2019.12.010
Tsukamoto T, Sakai E, Iizuka S, Taracena-Gándara M, Sakurai F, Mizuguchi H (2018) Generation of the adenovirus vector-mediated CRISPR/Cpf1 system and the application for primary human hepatocytes prepared from humanized mice with chimeric liver. Biol Pharm Bull 41(7):1089–1095. https://doi.org/10.1248/bpb.b18-00222
Lin CY, Chang YH, Li KC, Lu CH, Sung LY, Yeh CL et al (2013) The use of ASCs engineered to express BMP2 or TGF-β3 within scaffold constructs to promote calvarial bone repair. Biomaterials 34(37):9401–9412. https://doi.org/10.1016/j.biomaterials.2013.08.051
Truong VA, Hsu MN, Kieu Nguyen NT, Lin MW, Shen CC, Lin C, Y.,& Hu, Y. C. (2019) CRISPRai for simultaneous gene activation and inhibition to promote stem cell chondrogenesis and calvarial bone regeneration. Nucleic Acids Res 47(13):e74–e74. https://doi.org/10.1093/nar/gkz267
Bester AC, Lee JD, Chavez A, Lee YR, Nachmani D, Vora S et al (2018) An integrated genome-wide CRISPRa approach to functionalize lncRNAs in drug resistance. Cell 173(3):649–664. https://doi.org/10.1016/j.cell.2018.03.052
Grinsell D, Keating CP (2014) Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res Int. https://doi.org/10.1155/2014/698256
Hsu MN, Liao HT, Truong VA, Huang KL, Yu FJ, Chen HH et al (2019) CRISPR-based activation of endogenous neurotrophic genes in adipose stem cell sheets to stimulate peripheral nerve regeneration. Theranostics 9(21):6099. https://doi.org/10.7150/thno.36790
Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F et al (2008) Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26(11):1276–1284. https://doi.org/10.1038/nbt.1503
Faravelli I, Riboldi G, Nizzardo M, Simone C, Zanetta C, Bresolin N et al (2014) Stem cell transplantation for amyotrophic lateral sclerosis: therapeutic potential and perspectives on clinical translation. Cell Mol Life Sci 71(17):3257–3268. https://doi.org/10.1007/s00018-014-1613-4
Csobonyeiova M, Polak S, Nicodemou A, Danisovic L (2017) Induced pluripotent stem cells in modeling and cell-based therapy of amyotrophic lateral sclerosis. J Physiol Pharmacol 68:649–657
Wang L, Yi F, Fu L, Yang J, Wang S, Wang Z et al (2017) CRISPR/Cas9-mediated targeted gene correction in amyotrophic lateral sclerosis patient iPSCs. Protein Cell 8(5):365–378. https://doi.org/10.1007/s13238-017-0397-3
Zhao C, Devlin AC, Chouhan AK, Selvaraj BT, Stavrou M, Burr K et al (2020) Mutant C9orf72 human iPSC-derived astrocytes cause non-cell autonomous motor neuron pathophysiology. Glia 68(5):1046–1064. https://doi.org/10.1002/glia.23761
Park CY, Lee DR, Sung JJ, Kim DW (2016) Genome-editing technologies for gene correction of hemophilia. Hum Genet 135(9):977–981. https://doi.org/10.1007/s00439-016-1699-x
Evens H, Chuah MK, VandenDriessche T (2018) Haemophilia gene therapy: from trailblazer to gamechanger. Haemophilia 24:50–59. https://doi.org/10.1111/hae.13494
Nelwan M (2017) Hemophilia A and induced pluripotent stem cells. J Adv Biol Biotechnol 14(3):1–11. https://doi.org/10.9734/JABB/2017/35111
Park CY, Kim DH, Son JS, Sung JJ, Lee J, Bae S et al (2015) Functional correction of large factor VIII gene chromosomal inversions in hemophilia A patient-derived iPSCs using CRISPR-Cas9. Cell Stem Cell 17(2):213–220. https://doi.org/10.1016/j.stem.2015.07.001
Fomin ME, Togarrati PP, Muench MO (2014) Progress and challenges in the development of a cell-based therapy for hemophilia A. J Thromb Haemost 12(12):1954–1965. https://doi.org/10.1111/jth.12750
Ramaswamy S, Tonnu N, Menon T, Lewis BM, Green KT, Wampler D (2018) Autologous and heterologous cell therapy for hemophilia B toward functional restoration of factor IX. Cell Rep 23(5):1565–1580. https://doi.org/10.1016/j.celrep.2018.03.121
Stephens CJ, Lauron EJ, Kashentseva E, Lu ZH, Yokoyama WM, Curiel DT (2019) Long-term correction of hemophilia B using adenoviral delivery of CRISPR/Cas9. J Controll Release 298:128–141. https://doi.org/10.1016/j.jconrel.2019.02.009
Lyu C, Shen J, Wang R, Gu H, Zhang J, Xue F et al (2018) Targeted genome engineering in human induced pluripotent stem cells from patients with hemophilia B using the CRISPR-Cas9 system. Stem Cell Res Ther 9(1):1–12. https://doi.org/10.1186/s13287-018-0839-8
Morishige S, Mizuno S, Ozawa H, Nakamura T, Mazahery A, Nomura K et al (2020) CRISPR/Cas9-mediated gene correction in hemophilia B patient-derived iPSCs. Int J Hematol 111(2):225–233. https://doi.org/10.1007/s12185-019-02765-0
Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A et al (2006) Correction of junctional epidermolysisbullosa by transplantation of genetically modified epidermal stem cells. Nat Med 12(12):1397–1402. https://doi.org/10.1038/nm1504
Webber BR, Osborn MJ, McElroy AN, Twaroski K, Lonetree CL, DeFeo AP et al (2016) CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysisbullosa. NPJ Regener Med 1(1):1–11. https://doi.org/10.1038/npjregenmed.2016.14
Bonafont J, Mencía Á, García M, Torres R, Rodríguez S, Carretero M et al (2019) Clinically relevant correction of recessive dystrophic epidermolysisbullosa by dual sgRNA CRISPR/Cas9-mediated gene editing. Mol Ther 27(5):986–998. https://doi.org/10.1016/j.ymthe.2019.03.007
Hainzl S, Peking P, Kocher T, Murauer EM, Larcher F, Del Rio M et al (2017) COL7A1 editing via CRISPR/Cas9 in recessive dystrophic epidermolysisbullosa. Mol Ther 25(11):2573–2584. https://doi.org/10.1016/j.ymthe.2017.07.005
Matre PR, Mu X, Wu J, Danila D, Hall MA, Kolonin MG et al (2019) CRISPR/Cas9-based dystrophin restoration reveals a novel role for dystrophin in bioenergetics and stress resistance of muscle progenitors. Stem Cells 37(12):1615–1628. https://doi.org/10.1002/stem.3094
Hagan M, Ashraf M, Kim IM, Weintraub NL, Tang Y (2018) Effective regeneration of dystrophic muscle using autologous iPSC-derived progenitors with CRISPR-Cas9 mediated precise correction. Med Hypotheses 110:97–100. https://doi.org/10.1016/j.mehy.2017.11.009
Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN (2014) Prevention of muscular dystrophy in mice by CRISPR/Cas9–mediated editing of germline DNA. Science 345(6201):1184–1188. https://doi.org/10.1126/science.1254445
Emami MR, Young CS, Ji Y, Liu X, Mokhonova E, AD Pyle et al (2019) Polyrotaxane nanocarriers can deliver CRISPR/Cas9 plasmid to dystrophic muscle cells to successfully edit the DMD gene. Adv Ther 2(7):1900061. https://doi.org/10.1002/adtp.201900061
Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX et al (2016) In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351(6271):407–411. https://doi.org/10.1126/science.aad5177
Roberston MJ, Raghunathan S, Potaman VN, Zhang F, Stewart MD, McConnell BK, Schwartz RJ (2020) CRISPR-Cas9–induced IGF1 gene activation as a tool for enhancing muscle differentiation via multiple isoform expression. FASEB J 34(1):555–570. https://doi.org/10.1096/fj.201901107RR
Zhang Y, Long C, Li H, McAnally JR, Baskin KK, Shelton JM, Olson EN (2017) CRISPR-Cpf1 correction of muscular dystrophy mutations in human cardiomyocytes and mice. Sci Adv 3(4):e1602814.https://doi.org/10.1126/sciadv.1602814
Fellows CR, Williams R, Davies IR, Gohil K, Baird DM, Fairclough J et al (2017) Characterisation of a divergent progenitor cell sub-populations in human osteoarthritic cartilage: the role of telomere erosion and replicative senescence. Sci Rep 7(1):1–11. https://doi.org/10.1038/srep41421
Schuette HB, Kraeutler MJ, McCarty EC (2017) Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid-to long-term clinical outcomes. Orthop J Sports Med. https://doi.org/10.1177/2325967117709250
D’Costa S, Rich MJ, Diekman BO (2020) Engineered cartilage from human chondrocytes with homozygous knockout of cell cycle inhibitor p21. Tissue Eng A 26(7–8):441–449. https://doi.org/10.1089/ten.TEA.2019.0214
Karlsen TA, Pernas PF, Staerk J, Caglayan S, Brinchmann JE (2016) Generation of IL1β-resistant chondrocytes using CRISPR-CAS genome editing. Osteoarthr Cartil 24:S325. https://doi.org/10.1016/j.joca.2016.01.581
Cao A, Kan YW (2013) The prevention of thalassemia. Cold Spring Harbor Perspect Med. https://doi.org/10.1101/cshperspect.a011775
Yang Y, Zhang X, Yi L, Hou Z, Chen J, Kou X, Gao S (2016) Native induced pluripotent stem cells generated from b-thalassemia fibroblasts allow efficient gene correction with CRISPR/Cas9. Stem Cells Transl Med. https://doi.org/10.5966/sctm.2015-0157
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F et al (2010) Transfusion independence and HMGA2 activation after gene therapy of humanβ-thalassaemia. Nature 467(7313):318–322
Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS (1985) Insertion of DNA sequences into the human chromosomal β-globin locus by homologous recombination. Nature 317(6034):230–234. https://doi.org/10.1038/317230a0
Xie F, Ye L, Chang JC, Beyer AI, Wang J, Muench MO, Kan YW (2014) Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res 24(9):1526–1533. https://doi.org/10.1101/gr.173427.114
Harambat J, Fargue S, Acquaviva C, Gagnadoux MF, Janssen F, Liutkus A et al (2010) Genotype–phenotype correlation in primary hyperoxaluria type 1: the p. Gly170Arg AGXT mutation is associated with a better outcome. Kidney international 77(5):443–449. https://doi.org/10.1038/ki.2009.435
Bergstralh EJ, Monico CG, Lieske JC, Herges RM, Langman CB, Hoppe B et al (2010) Transplantation outcomes in primary hyperoxaluria. Am J Transpl 10(11):2493–2501. https://doi.org/10.1111/j.1600-6143.2010.03271.x
Salido EC, Li XM, Lu Y, Wang X, Santana A, Roy-Chowdhury N et al (2006) Alanine–glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc Natl Acad Sci USA 103(48):18249–18254. https://doi.org/10.1073/pnas.0607218103
Squires JE, Soltys KA, McKiernan P, Squires RH, Strom SC, Fox IJ, Soto-Gutierrez A (2017) Clinical hepatocyte transplantation: what is next? Curr Trans Rep 4(4):280–289. https://doi.org/10.1007/s40472-017-0165-6
Mc Kiernan PJ (2017) Recent advances in liver transplantation for metabolic disease. J Inherit Metab Dis 40(4):491–495. https://doi.org/10.1007/s10545-017-0020-z
Estève J, Blouin JM, Lalanne M, Azzi-Martin L, Dubus P, Bidet A et al (2019) Targeted gene therapy in human-induced pluripotent stem cells from a patient with primary hyperoxaluria type 1 using CRISPR/Cas9 technology. Biochem Biophys Res Commun 517(4):677–683. https://doi.org/10.1016/j.bbrc.2019.07.109
Estève J, Blouin JM, Lalanne M, Azzi-Martin L, Dubus P, Bidet A et al (2019) Generation of induced pluripotent stem cells-derived hepatocyte-like cells for ex vivo gene therapy of primary hyperoxaluria type 1. Stem Cell Res. https://doi.org/10.1016/j.scr.2019.101467
Dekkers JF, Wiegerinck CL, De Jonge HR, Bronsveld I, Janssens HM, De Winter-de Groot KM et al (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19(7):939–945. https://doi.org/10.1038/nm.3201
Riordan JR, Rommens JM, Kerem BS, Alon N, Rozmahel R, Grzelczak Z et al (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245(4922):1066–1073. https://doi.org/10.1126/science.2475911
Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E et al (2015) Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 12(9):1385–1390. https://doi.org/10.1016/j.celrep.2015.07.062
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T et al (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13(6):653–658. https://doi.org/10.1016/j.stem.2013.11.002
Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML et al (2015) Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Rep 4(4):569–577. https://doi.org/10.1016/j.stemcr.2015.02.005
Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK et al (2021) CRISPR-Cas9 gene editing for sickle cell disease andβ-thalassemia. N Engl J Med 384(3):252–260. https://doi.org/10.1056/NEJMoa2031054
Wei T, Cheng Q, Farbiak L, Anderson DG, Langer R, Siegwart DJ (2020) Delivery of tissue-targeted scalpels: opportunities and challenges for in vivo CRISPR/Cas-based genome editing. ACS Nano 14(8):9243–9262. https://doi.org/10.1021/acsnano.0c04707
Zhao J, Song Y, Liu D (2019) Clinical trials of dual-target CAR T cells, donor-derived CAR T cells, and universal CAR T cells for acute lymphoid leukemia. J Hematol Oncol 12(1):1–11. doi:https://doi.org/10.1186/s13045-019-0705-x
Karapurkar JK, Antao AM, Kim KS, Ramakrishna S (2021) CRISPR-Cas9 based genome editing for defective gene correction in humans and other mammals. Prog Mol Biol Transl Sci 181:185–229. https://doi.org/10.1016/bs.pmbts.2021.01.018
Mckiver B, Damaj MI, Sarkar D (2020) Assessment of current gene therapy practices in hepatocellular carcinoma. Gastrointest Disord 2(4):469–480. https://doi.org/10.3390/gidisord2040042
Lai Y, Babunovic GH, Cui L, Dedon PC, Doench JG, Fortune SM, Lu TK (2020) Illuminating host-mycobacterial interactions with genome-wide crispr knockout and CRISPRi screens. Cell Syst 11(3):239–251. https://doi.org/10.1016/j.cels.2020.08.010
Moghadam F, LeGraw R, Velazquez JJ, Yeo NC, Xu C, Park J et al (2020) Synthetic immunomodulation with a CRISPR super-repressor in vivo. Nat Cell Biol 22(9):1143–1154. https://doi.org/10.1038/s41556-020-0563-3
Hendriks D, Artegiani B, Hu H, de Sousa Lopes SC, Clevers H (2021) Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat Protoc 16(1):182–217. doi:https://doi.org/10.1038/s41596-020-00411-2
Acknowledgements
Authors are thankful to The Management of Sri Venkateswara College of Engineering, Sriperumbudur, Chennai, Tamil Nadu, India, Management of Sree Sastha Institute of Engineering and Technology, Chembarambakkam, Chennai, Tamil Nadu, India and The Management, Vice Chancellor, Dean of SMNS and Head of Biological Sciences, The Copperbelt University, Kitwe, Zambia for their constant support to complete the review article.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SDK, MA and GK: literature collection, writing—original draft, Methodology, Resources. RS and MS: conceptualization, supervision, validation, writing—review & editing for the final version manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dilip Kumar, S., Aashabharathi, M., KarthigaDevi, G. et al. Insights of CRISPR-Cas systems in stem cells: progress in regenerative medicine. Mol Biol Rep 49, 657–673 (2022). https://doi.org/10.1007/s11033-021-06832-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06832-w