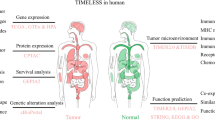
Abstract
Circadian rhythm is a periodic change of organism according to the law of external environment, which is manifested in metabolism, cell proliferation, physiology and behavior. In recent years, the role of circadian genes in the occurrence and progression of hematological malignancies have been continuously demonstrated. PER2 is the core component of the circadian rhythm playing an important role in regulating the circadian rhythm of the biological clock. This review summarizes the research progress of PER2 in hematological malignancies, especially leukemia, in order to better understand its role in hematological malignancies, and provide new ideas for clinical diagnosis and treatment.

Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Albrecht U (2012) Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 74(2):246–260. https://doi.org/10.1016/j.neuron.2012.04.006
Kim P, Oster H, Lehnert H et al (2019) Coupling the Circadian Clock to Homeostasis: The Role of Period in Timing Physiology. Endocr Rev 40(1):66–95. https://doi.org/10.1210/er.2018-00049
Partch CL, Green CB, Takahashi JS (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24(2):90–99. https://doi.org/10.1016/j.tcb.2013.07.002
Man AWC, Li H, Xia N (2021) Circadian rhythm: potential therapeutic target for atherosclerosis and thrombosis. Int J Mol Sci. https://doi.org/10.3390/ijms22020676
Mohawk JA, Green CB, Takahashi JS (2012) Central and Peripheral Circadian Clocks in Mammals. Annu Rev Neurosci 35(1):445–462. https://doi.org/10.1146/annurev-neuro-060909-153128
Ch R, Rey G, Ray S et al (2021) Rhythmic glucose metabolism regulates the redox circadian clockwork in human red blood cells. Nat Commun 12(1):377. https://doi.org/10.1038/s41467-020-20479-4
Ikegami K, Refetoff S, Van Cauter E et al (2019) Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol 15(10):590–600. https://doi.org/10.1038/s41574-019-0237-z
Kettner NM, Katchy CA, Fu L (2014) Circadian gene variants in cancer. Ann Med 46(4):208–220. https://doi.org/10.3109/07853890.2014.914808
Masri S, Sassone-Corsi P (2018) The emerging link between cancer, metabolism, and circadian rhythms. Nat Med 24(12):1795–1803. https://doi.org/10.1038/s41591-018-0271-8
Deshantri AK, Varela Moreira A, Ecker V et al (2018) Nanomedicines for the treatment of hematological malignancies. J Control Release 287:194–215. https://doi.org/10.1016/j.jconrel.2018.08.034
Bargiello TA, Jackson FR, Young MW (1984) Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312(5996):752–754. https://doi.org/10.1038/312752a0
Zhu L, Yu J, Zhang W et al (2014) Research progress on the central mechanism underlying regulation of visceral biological rhythm by per2. Mol Med Rep 10(5):2241–2248. https://doi.org/10.3892/mmr.2014.2559
Bargiello TA, Young MW (1984) Molecular genetics of a biological clock in Drosophila. Proc Natl Acad Sci USA 81(7):2142–2146. https://doi.org/10.1073/pnas.81.7.2142
Reddy P, Zehring WA, Wheeler DA et al (1984) Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38(3):701–710. https://doi.org/10.1016/0092-8674(84)90265-4
Kim M, de la Peña JB, Cheong JH et al (2018) Neurobiological functions of the period circadian clock 2 gene, Per2. Biomol Ther 26(4):358–367. https://doi.org/10.4062/biomolther.2017.131
Kucera N, Schmalen I, Hennig S et al (2012) Unwinding the differences of the mammalian PERIOD clock proteins from crystal structure to cellular function. Proc Natl Acad Sci USA 109(9):3311–3316. https://doi.org/10.1073/pnas.1113280109
Patke A, Young MW, Axelrod S (2019) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21(2):67–84. https://doi.org/10.1038/s41580-019-0179-2
Chiou Y-Y, Li T-Y, Yang Y et al (2020) A Sextuple Knockout Cell Line System to Study the Differential Roles of CRY, PER, and NR1D in the Transcription-Translation Feedback Loop of the Circadian Clock. Front NeuroSci 14:616802. https://doi.org/10.3389/fnins.2020.616802
Cao X, Yang Y, Selby CP et al (2021) Molecular mechanism of the repressive phase of the mammalian circadian clock. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2021174118
Fu L, Kettner NM (2013) The circadian clock in cancer development and therapy. Prog Mol Biol Transl Sci 119:221–282. https://doi.org/10.1016/B978-0-12-396971-2.00009-9
van der Horst GT, Muijtjens M, Kobayashi K et al (1999) Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398(6728):627–630. https://doi.org/10.1038/19323
Takahashi JS (2015) Molecular components of the circadian clock in mammals. Diabetes Obes Metab 17:6–11. https://doi.org/10.1111/dom.12514
Preitner N, Damiola F, Lopez-Molina L et al (2002) The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110(2):251–260. https://doi.org/10.1016/s0092-8674(02)00825-5
Chen ST, Choo KB, Hou MF et al (2005) Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis 26(7):1241–1246. https://doi.org/10.1093/carcin/bgi075
Albrecht U, Bordon A, Schmutz I et al (2007) The multiple facets of Per2. Cold Spring Harb Symp Quant Biol 72:95–104. https://doi.org/10.1101/sqb.2007.72.001
Miki T, Matsumoto T, Zhao Z et al (2013) p53 regulates Period2 expression and the circadian clock. Nat Commun. https://doi.org/10.1038/ncomms3444
Chen J (2016) The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb Perspect Med 6(3):a026104. https://doi.org/10.1101/cshperspect.a026104
Gotoh T, Vila-Caballer M, Santos CS et al (2014) The circadian factor Period 2 modulates p53 stability and transcriptional activity in unstressed cells. Mol Biol Cell 25(19):3081–3093. https://doi.org/10.1091/mbc.E14-05-0993
Berns K, Hijmans EM, Mullenders J et al (2004) A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428(6981):431–437. https://doi.org/10.1038/nature02371
Lee CC (2006) Tumor suppression by the mammalian Period genes. Cancer Causes Control 17(4):525–530. https://doi.org/10.1007/s10552-005-9003-8
Fu L, Pelicano H, Liu J et al (2002) The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111(1):41–50. https://doi.org/10.1016/s0092-8674(02)00961-3
Shaashua L, Mayer S, Lior C et al (2020) Stromal expression of the core clock gene period 2 is essential for tumor initiation and metastatic colonization. Front Cell Dev Biol 8:587697. https://doi.org/10.3389/fcell.2020.587697
Zhang J, Lv H, Ji M et al (2020) Low circadian clock genes expression in cancers: A meta-analysis of its association with clinicopathological features and prognosis. PLoS One 15(5):e0233508. https://doi.org/10.1371/journal.pone.0233508
Papp SJ, Huber AL, Jordan SD et al (2015) DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization. Elife. https://doi.org/10.7554/eLife.04883
Peek CB, Levine DC, Cedernaes J et al (2017) Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metabol 25(1):86–92. https://doi.org/10.1016/j.cmet.2016.09.010
Wallach T, Schellenberg K, Maier B et al (2013) Dynamic circadian protein-protein interaction networks predict temporal organization of cellular functions. PLoS Genet 9(3):e1003398. https://doi.org/10.1371/journal.pgen.1003398
Bode B, Taneja R, Rossner MJ et al (2011) Advanced light-entrained activity onsets and restored free-running suprachiasmatic nucleus circadian rhythms in per2/dec mutant mice. Chronobiol Int 28(9):737–750. https://doi.org/10.3109/07420528.2011.607374
Kawamoto T, Noshiro M, Sato F et al (2004) A novel autofeedback loop of Dec1 transcription involved in circadian rhythm regulation. Biochem Biophys Res Commun 313(1):117–124
Griffin EA Jr, Staknis D, Weitz CJ (1999) Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286(5440):768–771. https://doi.org/10.1126/science.286.5440.768
Masuda S, Narasimamurthy R, Yoshitane H et al (2020) Mutation of a PER2 phosphodegron perturbs the circadian phosphoswitch. Proc Natl Acad Sci USA 117(20):10888–10896. https://doi.org/10.1073/pnas.2000266117
Knippschild U, Milne DM, Campbell LE et al (1997) p53 is phosphorylated in vitro and in vivo by the delta and epsilon isoforms of casein kinase 1 and enhances the level of casein kinase 1 delta in response to topoisomerase-directed drugs. Oncogene 15(14):1727–1736. https://doi.org/10.1038/sj.onc.1201541
Yang WS, Stockwell BR (2008) Inhibition of casein kinase 1-epsilon induces cancer-cell-selective, PERIOD2-dependent growth arrest. Genome Biol 9(6):R92. https://doi.org/10.1186/gb-2008-9-6-r92
Huttlin EL, Bruckner RJ, Paulo JA et al (2017) Architecture of the human interactome defines protein communities and disease networks. Nature 545(7655):505–509. https://doi.org/10.1038/nature22366
Eng GWL, Virshup DM (2017) Site-specific phosphorylation of casein kinase 1 δ (CK1δ) regulates its activity towards the circadian regulator PER2. PloS One 12(5):e0177834. https://doi.org/10.1371/journal.pone.0177834
Kamagata M, Ikeda Y, Sasaki H et al (2017) Potent synchronization of peripheral circadian clocks by glucocorticoid injections in PER2::LUC-Clock/Clock mice. Chronobiol Int 34(8):1067–1082. https://doi.org/10.1080/07420528.2017.1338716
Vielhaber E, Eide E, Rivers A et al (2000) Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol Cell Biol 20(13):4888–4899. https://doi.org/10.1128/mcb.20.13.4888-4899.2000
Tamiya H, Ogawa S, Ouchi Y et al (2016) Rigid cooperation of Per1 and Per2 proteins. Sci Rep 6:32769. https://doi.org/10.1038/srep32769
Asher G, Reinke H, Altmeyer M et al (2010) Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142(6):943–953. https://doi.org/10.1016/j.cell.2010.08.016
Fustin J-M, Doi M, Yamaguchi Y et al (2013) RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 155(4):793–806. https://doi.org/10.1016/j.cell.2013.10.026
Levine DC, Hong H, Weidemann BJ et al (2020) NAD controls circadian reprogramming through PER2 nuclear translocation to counter aging. Mol Cell. https://doi.org/10.1016/j.molcel.2020.04.010
Dierickx P, Van Laake LW, Geijsen N (2018) Circadian clocks: from stem cells to tissue homeostasis and regeneration. EMBO Rep 19(1):18–28. https://doi.org/10.15252/embr.201745130
Romerowicz-Misielak M, Kozioł K, Nowak S et al (2020) Altered dynamics in the circadian oscillation of clock genes in serum-shocked NIH-3T3 cells by the treatment of GYY4137 or AOAA. Arch Biochem Biophys 680:108237. https://doi.org/10.1016/j.abb.2019.108237
Sasaki H, Hokugo A, Wang L et al (2020) Neuronal PAS domain 2 (Npas2)-deficient fibroblasts accelerate skin wound healing and dermal collagen reconstruction. Anat Rec (Hoboken, NJ : 2007) 303(6):1630–1641. https://doi.org/10.1002/ar.24109
Lo Iacono M, Signorino E, Petiti J et al (2021) Genetic screening for potential new targets in chronic myeloid leukemia based on drosophila transgenic for human BCR-ABL1. Cancers (Basel). https://doi.org/10.3390/cancers13020293
Yang M-Y, Yang W-C, Lin P-M et al (2011) Altered expression of circadian clock genes in human chronic myeloid leukemia. J Biol Rhythms 26(2):136–148. https://doi.org/10.1177/0748730410395527
Yang MY, Chang JG, Lin PM et al (2006) Downregulation of circadian clock genes in chronic myeloid leukemia: alternative methylation pattern of hPER3. Cancer Sci 97(12):1298–1307. https://doi.org/10.1111/j.1349-7006.2006.00331.x
Gery S, Komatsu N, Kawamata N et al (2007) Epigenetic silencing of the candidate tumor suppressor gene Per1 in non-small cell lung cancer. Clin Cancer Res 13(5):1399–1404. https://doi.org/10.1158/1078-0432.Ccr-06-1730
Koch A, Joosten SC, Feng Z et al (2018) Analysis of DNA methylation in cancer: location revisited. Nat Rev Clin Oncol 15(7):459–466. https://doi.org/10.1038/s41571-018-0004-4
Hoffman AE, Yi CH, Zheng T et al (2010) CLOCK in breast tumorigenesis: genetic, epigenetic, and transcriptional profiling analyses. Cancer Res 70(4):1459–1468. https://doi.org/10.1158/0008-5472.Can-09-3798
Wang Z, Wang H, Guo H et al (2020) The circadian rhythm and core gene Period2 regulate the chemotherapy effect and multidrug resistance of ovarian cancer through the PI3K signaling pathway. Biosci Rep. https://doi.org/10.1042/bsr20202683
Sun CM, Huang SF, Zeng JM et al (2010) Per2 inhibits k562 leukemia cell growth in vitro and in vivo through cell cycle arrest and apoptosis induction. Pathol Oncol Res 16(3):403–411. https://doi.org/10.1007/s12253-009-9227-0
Wang N, Mi M, Wei X et al (2020) Circadian clock gene Period2 suppresses human chronic myeloid leukemia cell proliferation. Exp Ther Med. https://doi.org/10.3892/etm.2020.9276
Liu J, Zhang Y, Huang H et al (2021) Recent advances in Bcr-Abl tyrosine kinase inhibitors for overriding T315I mutation. Chemical Biol Drug Des 97(3):649–664. https://doi.org/10.1111/cbdd.13801
Li J, Yang F, Feng JK et al (2015) Expression of per2 gene in CML and its relationship with bcr/abl. Chin J Lab Diagn 19(9):1481–1483
Li Z, Philip M, Ferrell PB (2020) Alterations of T-cell-mediated immunity in acute myeloid leukemia. Oncogene 39(18):3611–3619. https://doi.org/10.1038/s41388-020-1239-y
Gery S, Gombart AF, Yi WS et al (2005) Transcription profiling of C/EBP targets identifies Per2 as a gene implicated in myeloid leukemia. Blood 106(8):2827–2836. https://doi.org/10.1182/blood-2005-01-0358
Scott LM, Civin CI, Rorth P et al (1992) A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood 80(7):1725–1735
Radomska HS, Huettner CS, Zhang P et al (1998) CCAAT/enhancer binding protein alpha is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol Cell Biol 18(7):4301–4314. https://doi.org/10.1128/mcb.18.7.4301
Gery S, Koeffler HP (2009) Per2 Is a C/EBP target gene implicated in myeloid leukemia. Integr Cancer Ther 8(4):317–320. https://doi.org/10.1177/1534735409352084
Albanesi J, Noguera NI, Banella C et al (2020) Transcriptional and metabolic dissection of ATRA-induced granulocytic differentiation in nb4 acute promyelocytic leukemia cells. Cells. https://doi.org/10.3390/cells9112423
Thomas X, Heiblig M (2020) Acute promyelocytic leukemia. Cancers (Basel). https://doi.org/10.3390/cancers12123718
Hoischen C, Monajembashi S, Weisshart K et al (2018) Multimodal light microscopy approaches to reveal structural and functional properties of promyelocytic leukemia nuclear bodies. Front Oncol 8:125. https://doi.org/10.3389/fonc.2018.00125
Miki T, Xu Z, Chen-Goodspeed M et al (2012) PML regulates PER2 nuclear localization and circadian function. EMBO J 31(6):1427–1439. https://doi.org/10.1038/emboj.2012.1
Miki T, Chen-Goodspeed M, Zhao Z et al (2013) Circadian behavior of mice deficient in PER1/PML or PER2/PML. J Circadian Rhythms 11(1):9. https://doi.org/10.1186/1740-3391-11-9
Zhu Y, Leaderer D, Guss C et al (2007) Ala394Thr polymorphism in the clock gene NPAS2: a circadian modifier for the risk of non-Hodgkin’s lymphoma. Int J Cancer 120(2):432–435. https://doi.org/10.1002/ijc.22321
Lahti TA, Partonen T, Kyyrönen P et al (2008) Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer 123(9):2148–2151. https://doi.org/10.1002/ijc.23566
Liu Y, Barta SK (2019) Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol 94(5):604–616. https://doi.org/10.1002/ajh.25460
Thoennissen NH, Thoennissen GB, Abbassi S et al (2012) Transcription factor CCAAT/enhancer-binding protein alpha and critical circadian clock downstream target gene PER2 are highly deregulated in diffuse large B-cell lymphoma. Leukemia Lymphoma 53(8):1577–1585. https://doi.org/10.3109/10428194.2012.658792
Lee HM, Chen R, Kim H et al (2011) The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc Natl Acad Sci USA 108(39):16451–16456. https://doi.org/10.1073/pnas.1107178108
Chun SK, Jang J, Chung S et al (2014) Identification and validation of cryptochrome inhibitors that modulate the molecular circadian clock. ACS Chem Biol 9(3):703–710. https://doi.org/10.1021/cb400752k
Rossi DJ, Bryder D, Seita J et al (2007) Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447(7145):725–729. https://doi.org/10.1038/nature05862
Rübe CE, Fricke A, Widmann TA et al (2011) Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One 6(3):e17487. https://doi.org/10.1371/journal.pone.0017487
Wang J, Morita Y, Han B et al (2016) Per2 induction limits lymphoid-biased haematopoietic stem cells and lymphopoiesis in the context of DNA damage and ageing. Nat Cell Biol 18(5):480–490. https://doi.org/10.1038/ncb3342
Wang J, Morita Y, Han B et al (2019) Author Correction: Per2 induction limits lymphoid-biased haematopoietic stem cells and lymphopoiesis in the context of DNA damage and ageing. Nat Cell Biol 21(6):791–792. https://doi.org/10.1038/s41556-019-0279-4
Tomita T, Kurita R, Onishi Y (2017) Epigenetic regulation of the circadian clock: role of 5-aza-2′-deoxycytidine. Biosci Rep. https://doi.org/10.1042/bsr20170053
Wolowiec D, Ciszak L, Kosmaczewska A et al (2001) Cell cycle regulatory proteins and apoptosis in B-cell chronic lymphocytic leukemia. Haematologica 86(12):1296–1304
Taniguchi H, Fernández AF, Setién F et al (2009) Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res 69(21):8447–8454. https://doi.org/10.1158/0008-5472.Can-09-0551
Rana S, Munawar M, Shahid A et al (2013) Deregulated expression of circadian clock and clock-controlled cell cycle genes in chronic lymphocytic leukemia. Mol Biol Rep 41(1):95–103. https://doi.org/10.1007/s11033-013-2841-7
D’Arena G, De Feo V, Pietrantuono G et al (2020) CD200 and Chronic Lymphocytic Leukemia: Biological and Clinical Relevance. Front Oncol 10:584427. https://doi.org/10.3389/fonc.2020.584427
Eisele L, Prinz R, Klein-Hitpass L et al (2009) Combined PER2 and CRY1 expression predicts outcome in chronic lymphocytic leukemia. Eur J Haematol 83(4):320–327. https://doi.org/10.1111/j.1600-0609.2009.01287.x
Dan H, Zhang S, Zhou Y et al (2019) DNA Methyltransferase Inhibitors: Catalysts For Antitumour Immune Responses. Onco Targets Ther 12:10903–10916. https://doi.org/10.2147/ott.S217767
Funding
This work was supported by Major scientific and technological innovation projects of Shandong Province of China (Grant No. 2019JZZY011018), Yantai Science and Technology Plan Project of China (Grant No. 2019MSGY133) and Yantai Science and Technology Plan Project of China (Grant No. 2019YD004).
Author information
Authors and Affiliations
Contributions
HJ and XY had the idea for the review and wrote the first draft of the manuscript. MM, XW performed the literature search. HW and YX collected data. CS critically revised the work. All authors have read and approved the manuscript, and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent for participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, H., Yang, X., Mi, M. et al. PER2: a potential molecular marker for hematological malignancies. Mol Biol Rep 48, 7587–7595 (2021). https://doi.org/10.1007/s11033-021-06751-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06751-w