Abstract
Background
Cisplatin has been extensively used in therapeutics for its broad-spectrum anticancer activity and frequently used for the treatment of solid tumors. However, it presents several side-effects and several cancers develop resistance. Combination therapy of cisplatin with poly (ADP-ribose) polymerase 1 (PARP1) inhibitors has been effective in increasing its efficacy at lower doses.
Methods and results
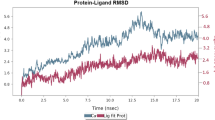
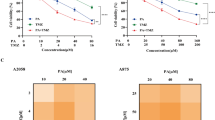
In this work, we have shown that the nitro-flavone derivative, 2-(4-Nitrophenyl)-4H-chromen-4-one (4NCO), can improve the sensitivity of cancer cells to cisplatin through inhibition of PARP1. The effect of 4NCO on cisplatin toxicity was studied through combination therapy in both exponential and density inhibited A375 melanoma cells. Combination index (CI) was determined from isobologram analysis. The mechanism of cell killing was assessed by lactate dehydrogenase (LDH) assay. Temporal nicotinamide adenine dinucleotide (NAD+) assay was done to show the inhibition of PARP1. We also performed in silico molecular modeling studies to know the binding mode of 4NCO to a modeled PARP1-DNA complex containing cisplatin-crosslinked adduct. The results from both in silico and in cellulo studies confirmed that PARP1 inhibition by 4NCO was most effective in sensitizing A375 melanoma cells to cisplatin. Isobologram analysis revealed that 4NCO reduced cell viability both in exponential and density inhibited A375 cells synergistically. The combination led to cell death through apoptosis.
Conclusion
The synthetic nitro-flavone derivative 4NCO effectively inhibited the important nuclear DNA repair enzyme PARP1 and therefore, could complement the DNA-damaging anticancer drug cisplatin in A375 cells and thus, could act as a potential adjuvant to cisplatin in melanoma therapy.





Similar content being viewed by others
Data availability
Data will be made available on request.
Code availability
Not Applicable.
References
Dasari S, Bernard Tchounwou P (2014) Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2014.07.025
Wang Z, Zhu G (2018) DNA Damage Repair Pathways and Repair of Cisplatin-Induced DNA Damage. Elsevier Inc, Amsterdam
Shamsi MH, Kraatz HB (2013) Interactions of metal ions with DNA and some applications. J Inorg Organomet Polym Mater 23:4–23. https://doi.org/10.1007/s10904-012-9694-8
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G (2012) Molecular mechanisms of cisplatin resistance. Oncogene 31:1869–1883. https://doi.org/10.1038/onc.2011.384
Mezencev R (2015) Interactions of cisplatin with non-DNA targets and their influence on anticancer activity and drug toxicity: the complex world of the platinum complex. Curr Cancer Drug Targets 14:794–816. https://doi.org/10.2174/1568009614666141128105146
Makovec T (2019) Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol 53:148–158. https://doi.org/10.2478/raon-2019-0018
Siddik ZH (2003) Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 22:7265–7279. https://doi.org/10.1038/sj.onc.1206933
Ma L, Wang H, Wang C, Su J, Xie Q, Xu L, Yu Y, Liu S, Li S, Xu Y, Li Z (2016) Failure of elevating calcium induces oxidative stress tolerance and imparts cisplatin resistance in ovarian cancer cells. Aging Dis 7:254–266. https://doi.org/10.14336/AD.2016.0118
Shen DW, Pouliot LM, Hall MD, Gottesman MM (2012) Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 64:706–721. https://doi.org/10.1124/pr.111.005637
Aldossary SA (2019) Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomed Pharmacol J 12:7–15. https://doi.org/10.13005/bpj/1608
Kubala M, Geleticova J, Huliciak M, Zatloukalova M, Vacek J, Sebela M (2014) Na+/K+-ATPase inhibition by cisplatin and consequences for cisplatin nephrotoxicity. Biomed Pap 158:194–200. https://doi.org/10.5507/bp.2014.018
Howell SB, Safaei R, Larson CA, Sailor MJ (2010) Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol 77:887–894. https://doi.org/10.1124/mol.109.063172.that
Chen Z-S, Tiwari AK (2011) Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J 278:3226–3245. https://doi.org/10.1111/j.1742-4658.2011.08235.x.Multidrug
Michels J, Vitale I, Galluzzi L, Adam J, Olaussen KA, Kepp O, Senovilla L, Talhaoui I, Guegan J, Enot DP, Talbot M, Robin A, Girard P, Orear C, Lissa D, Sukkurwala AQ, Garcia P, Behnam-Motlagh P, Kohno K, Wu GS, Brenner C, Dessen P, Saparbaev M, Soria JC, Castedo M, Kroemer G (2013) Cisplatin resistance associated with PARP hyperactivation. Cancer Res 73:2271–2280. https://doi.org/10.1158/0008-5472.CAN-12-3000
Tanida S, Mizoshita T, Ozeki K, Tsukamoto H, Kamiya T, Kataoka H, Sakamuro D, Joh T (2012) Mechanisms of cisplatin-induced apoptosis and of cisplatin sensitivity: Potential of BIN1 to act as a potent predictor of cisplatin sensitivity in gastric cancer treatment. Int J Surg Oncol. https://doi.org/10.1155/2012/862879
Basu A, Krishnamurthy S (2010) Cellular responses to cisplatin-induced DNA damage. J Nucleic Acids. https://doi.org/10.4061/2010/201367
Karmakar S, Manna D, Ghosh S, Hansda S, Mitra A, Bagchi A, Ghosh R (2017) 9-Phenyl acridine: a possible poly ( ADP-ribose ) polymerase-1 inhibitor. J Chem Pharm Res 9:188–200
Karmakar S, Ghosh R (2018) Synergistic action of cisplatin and 9-phenyl acridine in A375 cells. Indian J Biochem Biophys 55:173–182
Geraets L, Moonen HJ, Brauers K, Wouters EF, Bast A, Hageman GJ (2007) Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J Nutr 137:2190–2195. https://doi.org/10.1093/jn/137.10.2190
Mitra A, Biswas R, Bagchi A, Ghosh R (2019) Insight into the binding of a synthetic nitro-flavone derivative with human poly (ADP-ribose) polymerase 1. Int J Biol Macromol 141:444–459. https://doi.org/10.1016/j.ijbiomac.2019.08.242
Cushman M, Nagarathnam D, Burg DL, Geahlen RL (1991) Synthesis and protein-tyrosine kinase inhibitory activities of flavonoid analogues. J Med Chem 34:798–806. https://doi.org/10.1021/jm00106a047
Sekhar PN, Kishor PBK, Zubaidha PK, Hashmi AM, Kadam TA, Anandareddy L, De MM, Kumar KP, Bhaskar BV, Munichandrababu T, Jayasree G, Narayana PVBS, Gyananath G (2011) Experimental validation and docking studies of flavone derivatives on aldose reductase involved in diabetic retinopathy, neuropathy, and nephropathy. Med Chem Res 20:930–945. https://doi.org/10.1007/s00044-010-9412-4
Nakanishi I, Murata K, Nagata N, Kurono M, Kinoshita T, Yasue M, Miyazaki T, Takei Y, Nakamura S, Sakurai A, Iwamoto N, Nishiwaki K, Nakaniwa T, Sekiguchi Y, Hirasawa A, Tsujimoto G, Kitaura K (2015) Identification of protein kinase CK2 inhibitors using solvent dipole ordering virtual screening. Eur J Med Chem 96:396–404. https://doi.org/10.1016/j.ejmech.2015.04.032
Wagal OS, Joshi AJ, Joshi UJ, Bhojwani HR, Begwani KV, Dawne HA, Gude RP, Sathaye SSKD (2021) Studies in molecular modeling, in-vitro CDK2 inhibition and antimetastatic activity of some synthetic flavones. Front Biosci (Landmark Ed) 26:664–681
Mitra A, Saikh F, Das J, Ghosh S, Ghosh R (2018) Studies on the interaction of a synthetic nitro-flavone derivative with DNA: A multi-spectroscopic and molecular docking approach. Spectrochim Acta Part A Mol Biomol Spectrosc 203:357–369. https://doi.org/10.1016/j.saa.2018.05.073
Masunaga S-I, Ono K, Abe M (1992) Potentially Lethal damage repair by quiescent cells in murine solid tumors. Int J Radiat Oncol 22:973–978
Huang RY, Pei L, Liu Q, Chen S, Dou H, Shu G, Yuan ZX, Lin J, Peng G, Zhang W, Fu H (2019) Isobologram analysis: a comprehensive review of methodology and current research. Front Pharmacol 10:1–12. https://doi.org/10.3389/fphar.2019.01222
Chou TC (2010) Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res 70:440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Manna D, Bhuyan R, Saikh F, Ghosh S, Basak J, Ghosh R (2018) Novel 1,4-dihydropyridine induces apoptosis in human cancer cells through overexpression of Sirtuin1. Apoptosis 23:532–553. https://doi.org/10.1007/s10495-018-1483-6
Ghosh R, Guha D, Bhowmik S, Karmakar S (2013) Antioxidant enzymes and the mechanism of the bystander effect induced by ultraviolet C irradiation of A375 human melanoma cells. Mutat Res Genet Toxicol Environ Mutagen 757:83–90. https://doi.org/10.1016/j.mrgentox.2013.06.022
Langelier M, Planck JL, Roy S, Pascal JM (2012) Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science 336:728–732. https://doi.org/10.1126/science.1216338
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, I.N. Shindyalov PEB, (2000) The protein data bank. Nucleic Acids Res 28:235–242. https://doi.org/10.1093/nar/28.1.235
Ramachandran GN, Ramakrishnan C, Sasisekharan V (1963) Stereochemistry of polypeptide chain configurations. J Mol Biol 7:95–99. https://doi.org/10.1016/S0022-2836(63)80023-6
Coste F, Malinge JM, Serre L, Shepard W, Roth M, Leng M, Zelwer C (1999) Crystal structure of a double-stranded DNA containing a cisplatin interstrand cross-link at 1.63 Å resolution: Hydration at the platinated site. Nucleic Acids Res 27:1837–1846. https://doi.org/10.1093/nar/27.8.1837
Yan Y, Zhang D, Zhou P, Li B, Huang SY (2017) HDOCK: A web server for protein-protein and protein-DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res 45:W365–W373. https://doi.org/10.1093/nar/gkx407
Tuszynska I, Magnus M, Jonak K, Dawson W, Bujnicki JM (2015) NPDock: A web server for protein-nucleic acid docking. Nucleic Acids Res 43:W425–W430. https://doi.org/10.1093/nar/gkv493
Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ (2005) PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res 33:363–367. https://doi.org/10.1093/nar/gki481
(2018) ACD/Chemsketch version 2018.1 Advanced Chemistry Development, Inc., Toronto, ON, Canada, www.acdlabs.com
The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC, DeLano WL (2002) Pymol An open-source Mol Graph tool. CCP4 Newsl Protein Crystallogr 40:82–92
Discovery Studio Visualizer Software, version 2.5. http://www.accelrys.com
Macindoe G, Mavridis L, Venkatraman V, Devignes MD, Ritchie DW (2010) HexServer: An FFT-based protein docking server powered by graphics processors. Nucleic Acids Res 38:445–449. https://doi.org/10.1093/nar/gkq311
Mann M, Kumar S, Sharma A, Chauhan SS, Bhatla N, Kumar S, Bakhshi S, Gupta R, Kumar L (2019) PARP inhibition enhances cisplatin sensitivity in cervical cancer by modulating β-catenin signaling. Ann Oncol 30:v19. https://doi.org/10.1093/annonc/mdz238.065
Cheng H, Zhang Z, Borczuk A, Powell CA, Balajee AS, Lieberman HB, Halmos B (2013) PARP inhibition selectively increases sensitivity to cisplatin in ERCC1-low non-small cell lung cancer cells. Carcinogenesis 34:739–749. https://doi.org/10.1093/carcin/bgs393
Wang L, Liang C, Li F, Guan D, Wu X, Fu X, Lu A, Zhang G (2017) PARP1 in carcinomas and PARP1 inhibitors as antineoplastic drugs. Int J Mol Sci 18:1–16. https://doi.org/10.3390/ijms18102111
Zheng YD, Xu XQ, Peng F, Yu JZ, Wu H (2011) The poly(ADP-ribose) polymerase-1 inhibitor 3-aminobenzamide suppresses cell growth and migration, enhancing suppressive effects of cisplatin in osteosarcoma cells. Oncol Rep 25:1399–1405. https://doi.org/10.3892/or.2011.1212
Swindall AF, Stanley JA, Yang ES (2013) PARP-1: Friend or foe of DNA damage and repair in tumorigenesis. Cancers (Basel) 5:943–958. https://doi.org/10.3390/cancers5030943
Mitra A, Bhowmik S, Ghosh R (2021) Preferential interaction with c-MYC quadruplex DNA mediates the cytotoxic activity of a nitro-flavone derivative in A375 cells. J Photochem Photobiol 6:100033. https://doi.org/10.1016/j.jpap.2021.100033
Song L, McNeil EM, Ritchie AM, Astell KR, Gourley C, Melton DW (2017) Melanoma cells replicate through chemotherapy by reducing levels of key homologous recombination protein RAD51 and increasing expression of translesion synthesis DNA polymerase ζ. BMC Cancer 17:1–14. https://doi.org/10.1186/s12885-017-3864-6
Kauffmann A, Rosselli F, Lazar V, Winnepenninckx V, Mansuet-Lupo A, Dessen P, Van Den Oord JJ, Spatz A, Sarasin A (2008) High expression of DNA repair pathways is associated with metastasis in melanoma patients. Oncogene 27:565–573. https://doi.org/10.1038/sj.onc.1210700
Song L, Robson T, Doig T, Brenn T, Mathers M, Brown ER, Doherty V, Bartlett JMS, Anderson N, Melton DW (2013) DNA repair and replication proteins as prognostic markers in melanoma. Histopathology 62:343–350. https://doi.org/10.1111/j.1365-2559.2012.04362.x
Jewell R, Conway C, Mitra A, Randerson-Moor J, Lobo S, Nsengimana J, Harland M, Marples M, Edward S, Cook M, Powell B, Boon A, De Kort F, Parker KA, Cree IA, Barrett JH, Knowles MA, Bishop DT, Newton-Bishop J (2010) Patterns of expression of DNA repair genes and relapse from melanoma. Clin Cancer Res 16:5211–5221. https://doi.org/10.1158/1078-0432.CCR-10-1521
Karwaciak I, Sałkowska A, Karaś K, Dastych J, Ratajewski M (2021) Targeting SIRT2 sensitizes melanoma cells to cisplatin via an EGFR-dependent mechanism. Int J Mol Sci 22:5034. https://doi.org/10.3390/ijms22095034
Campagna R, Bacchetti T, Salvolini E, Pozzi V, Molinelli E, Brisigotti V, Sartini D, Campanati A, Ferretti G, Annamaria O, Emanuelli M (2020) Paraoxonase-2 silencing enhances sensitivity of A375 melanoma cells to treatment with cisplatin. Antioxidants 9:1238. https://doi.org/10.3390/antiox9121238
Quezada MJ, Picco ME, Villanueva MB, Castro MV, Barbero G, Fernández NB, Illescas E, Lopez-Bergami P (2021) BCL2L10 Is overexpressed in melanoma downstream of STAT3 and promotes cisplatin and ABT-737 resistance. Cancers (Basel) 13:78. https://doi.org/10.3390/cancers13010078
Singleton KR, Crawford L, Tsui E, Manchester HE, Maertens O, Liu X, Liberti MV, Magpusao AN, Stein EM, Tingley JP, Frederick DT, Boland GM, Flaherty KT, McCall SJ, Krepler C, Sproesser K, Herlyn M, Adams DJ, Locasale JW, Cichowski K, Mukherjee S, Wood KC (2017) Melanoma therapeutic strategies that select against resistance by exploiting MYC-driven evolutionary convergence. Cell Rep 21:2796–2812. https://doi.org/10.1016/j.celrep.2017.11.022
Malyuchenko NV, Kotova EY, Kulaeva OI, Kirpichnikov MP, Studitskiy VM (2015) PARP1 inhibitors: antitumor drug design. Acta Naturae 7:27–37
Karam AK, Santiskulvong C, Fekete M, Zabih S, Eng C, Dorigo O (2010) Cisplatin and PI3kinase inhibition decrease invasion and migration of human ovarian carcinoma cells and regulate matrix-metalloproteinase expression. Cytoskeleton 67:535–544. https://doi.org/10.1002/cm.20465
Ghosh R, Bhattacharjee SB (1990) Killing and mutation induction in quiescent V79 cells as influenced by nalidixic acid. Mutat Res Lett 243:7–12. https://doi.org/10.1016/0165-7992(90)90116-2
Florea AM, Büsselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 3:1351–1371. https://doi.org/10.3390/cancers3011351
Ghosh (Datta) R, Bhattacharjee SB, (1989) Influence of benzamide on killing and mutation of density-inhibited V79 cells by MNNG. Mutat Res Lett 225:137–141. https://doi.org/10.1016/0165-7992(89)90131-0
Curtin NJ (2008) PARP Inhibitors and Cancer Therapy. In: Curtin NJ (ed) Poly(ADP-Ribosyl)ation. Springer, Boston, pp 218–233
Kelley SK, Ashkenazi A (2004) Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol 4:333–339. https://doi.org/10.1016/j.coph.2004.02.006
Ghosh R, Girigoswami K, Guha D (2012) Suppression of apoptosis leads to cisplatin resistance in V79 cells subjected to chronic oxidative stress. Indian J Biochem Biophys 49:363–370
Acknowledgements
The authors are grateful to Prof. S. Ghosh, Department of Chemistry, Jadavpur University for providing authentic samples of 4NCO for the work. The author, Anindita Mitra, acknowledges fellowship from Department of Science and Technology (DST-INSPIRE Grant: DST/INSPIRE Fellowship/2015/IF150061), Govt. of India. The authors also acknowledge the infrastructural facility at Department of Biotechnology (DBT) sponsored Bioinformatics Infrastructure Facility (BIF Centre), University of Kalyani for the work.
Funding
Department of Science & Technology—Promotion of University Research and Scientific Excellence II (DST-PURSE II), Govt. of India and University Grants Commission: Special Assistance Programme Departmental Research Support II (UGC-SAP DRS II), Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Ethical approval
Not Applicable; Studies were on cultured cells.
Consent to participate
Not Applicable.
Consent for publication
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mitra, A., Ghosh, R. A375 melanoma cells are sensitized to cisplatin-induced toxicity by a synthetic nitro-flavone derivative 2-(4-Nitrophenyl)-4H-chromen-4-one through inhibition of PARP1. Mol Biol Rep 48, 5993–6005 (2021). https://doi.org/10.1007/s11033-021-06600-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06600-w