Abstract
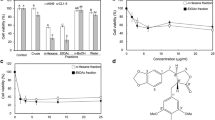
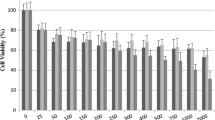
Cancer is a public health problem in the world accounting for most of the deaths. Currently, common treatment of cancer such as chemotherapy works by killing fast-growing cancer cells. Unfortunately, chemotherapy cannot tell the difference between cancer cells and fast-growing healthy cells, including red and white blood cells. As a result, one of the most serious potential side effects of some types of chemotherapy is a low white blood cell count that makes it unreliable (Parkin et al. [34]; Pauk et al. [3]). Even though intense research has been going on in recent years, successful therapeutic targets against this disease have been elusive. In this study, we evaluate the anti-proliferative activity of Euphorbia mauritanica and Kedrostis hirtella in lung cancer. In our assessment it was observed that E. mauritanica and K. hirtella were able to induce cell death at 5 μg/ml in A549 cells over 22 h and at 10 μg/ml over 24 h in the Lqr1 cell line. Molecular analysis of DNA fragmentation and Annexin V were used to examine the type of cell death induced by E. mauritanica and K. hirtella extracts. These results showed an increase in necrotic and apoptotic characteristics with both nuclear DNA fragmentation and smear. Therefore, these results suggest that E. mauritanica and K. hirtella may play a role in inducing cell death in lung cancer cells. However, further studies need to be conducted to ascertain these results.






Similar content being viewed by others
References
Bradshaw D, Groenewald P, Laubcher R. (2003) Initial Burden of disease Estimates for South Africa, 2000. South African Medical Research Council, Cape Town
Massion PP, Carbone DP (2003) The molecular basis of lung cancer: molecular abnormalities and therapeutic implications. Respir Res 4:12–13
Pauk N, Kubik A, Zatloukal P, Krepela E (2005) Lung cancer in women. Lung Cancer 48:1–9
Motadi LR, Bhoola KD, Dlamini Z (2011) Expression and function of retinoblastoma binding protein 6 (RBBP6) in human lung cancer. Immunobiology 216(10):1065–1073
Hecht A, Litterst CM, Huber O, Kemler R (1999) Functional characterization of multiple transactivating elements in β-catenin some of which interact with the TATA binding protein in vitro. J Biol Chem 274:18017–18025
Cragg GM, Newman D (1999) Discovery and development of antineoplastic agents from natural sources. Cancer Investig 17:153–163
Mann J (2002) Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer 2:143–148
Ferguson LR, Gourdie TA, Valu KK, Denny WA (1989) Bacterial mutagenicity studies of DNA-intercalating aniline mustards: an insight into the mode of action of a novel class of antitumour drugs. Anticancer Drug Des 4:209–216
Ooi LK, Muhammad TST, Sulaiman F (2010) Growth arrest and induction of apoptotic and non-apoptotic programmed cell death by, Physalis minima L. chloroform extract in human ovarian carcinoma Caov-3 cells. J Ethnopharmacol 128(1):92–99
Chinkwo KA (2005) Sutherlandia frutescens extracts can induce apoptosis in cultured carcinoma cells. J Ethnopharmacol 98(1–2):163–164
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Masoko P, Picard J, Eloff JN (2005) Antifungal activities of six South African Terminalia species (Combretaceae). J Ethnopharmacol 99:301–308
Carmichael WW, Beasley V, Bunner DL, JN Eloff, Falconer IR et al (1988) Naming of cyclic heptapeptide toxins of cyanobacteria (blue-green algae). Toxicon 26:971–973
Sawamura M, Odajima K, Nagakura K, Nakamura H (1989) Chemosensitivity testing on human bladder cancer cell lines, using MTT-assay. Nihon Hinyokika Gakkai Zasshi 80(8):1195–1202
Mani SWC, Francis R, Pestell RG (2000) Cyclin-dependent kinase inhibitors: novel anti-cancer drugs. Exp Opin Investig Drugs 9(10):1849–1870
Ueda T, Tamaki M, Ogawa O, Yoshimura N (2002) Over expression of platelet-derived endothelial cell growth factor/thymidine phosphorylase in patients with interstitial cystitis and bladder carcinoma. J Urol 167(1):347–351
Creemers GJ, Bolis G, Gore M, Scarfone G, Lacave AJ, Guastalla JP et al (1996) Topotecan, an active drug in the second-line treatment of epithelial ovarian cancer. J Clin Oncol 14:3056–3061
Kummalue T, Chuphrom A, Sukpanichanant S, Pongpruttipan T, Sukpanichanant S (2010) Detection of monoclonal immunoglobulin heavy chain gene rearrangement (FR3) in Thai malignant lymphoma by high resolution melting curve analysis. Diagn Pathol 19(5):31
Saleem A, Husheem M, Härkönen P, Pihlaja K (2002) Inhibition of cancer cell growth by crude extract and the phenolic of Terminalia chebula retz. fruit. J Ethnopharmacol 81(3):327–336
Zhao S, Du X, Chai M, Chen J, Zhou Y, Song J (2002) Secretory phospholipase A2 induces apoptosis via a mechanism involving ceramide generation. Biochim Biophys Acta 1581:75–88
Kroemer G, Dallaporta B, Resche-Rigon M (1998) The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol 60:619–642
Ren Y, Savill J (1998) Apoptosis: the importance of being eaten. Cell Death Differ 5(7):563–568
Chowdhury G, Guengerich FP (2009) Tandem mass spectrometry-based detection of c4′-oxidized abasic sites at specific positions in DNA fragments. Chem Res Toxicol 22(7):1310–1319
Zhivotosky B, Orrenius S (2001) Assessment of apoptosis and necrosis by DNA fragmentation and morphological criteria. Curr Protoc Cell Biol 1–18.3
Williamson DH, Krebs HA, Stubbs M, Page MA, Morris HP, Weber G (1970) Metabolism of renal tumors in situ and during ischemia. Cancer Res 30(7):2049–2054
Skalka M, Matyásová J, Cejková M (1976) Dna in chromatin of irradiated lymphoid tissues degrades in vivo into regular fragments. FEBS Lett 72(2):271–274
Koopman G, Reutelingsperger CPM, Kuiljten G, Keehnen RMJ, Pals ST, Van Oers MHJ (1994) Annexin V for flow cytometric detection of phosphatidylserine. Expression on B cells undergoing apoptosis. Blood 84:1415–1420
Vermes I, Haanen C, Steffen-Nakken H, Reutelingsperger C (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Method 184:39–51
Lecoeur H (2002) Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases. Exp Cell Res 277(1):1–14
Gao S, Scott RE (2003) Stable overexpression of specific segments of the P2P-R protein in human MCF-7 cells promotes camptothecin-induced apoptosis. J Cell Physiol 197(3):445–452
Moll UM, Petrenko O (2003) The MDM2–p53 interaction. Mol Cancer Res 1(14):1001–1008
Yoshitake J, Akaike T, Akuta T, Tamura F, Ogura T, Esumi H, Maeda H (2004) Nitric oxide as an endogenous mutagen for Sendai virus without antiviral activity. J Virol 78(16):8709–8719
Chibi M, Meyer M, Skepu A, Rees GDJ, Moolman-Smook JC, Pugh DJ (2008) RBBP6 interacts with multifunctional protein YB-1 through its RING finger domain, leading to ubiquitination and proteosomal degradation of YB-1. J Mol Biol 384(4):908–916
Parkin DM, Pisani P, Ferlay J (1993) Estimates of worldwide incidence of eighteen major cancers in 1985. Int J Cancer 54:594–606
Acknowledgments
This work was supported by grants from the National Research Foundation of South Africa and the University of the Witwatersrand Research Council. We would further like to thank Dr. Mokgoto from the University of Limpopo and Prof. Newman for their assistance with some methods.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thafeni, M.A., Sayed, Y. & Motadi, L.R. Euphorbia mauritanica and Kedrostis hirtella extracts can induce anti-proliferative activities in lung cancer cells. Mol Biol Rep 39, 10785–10794 (2012). https://doi.org/10.1007/s11033-012-1972-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1972-6