Abstract
The objective of this study was to estimate the impact of the polymorphism of μ-calpain (CAPN1S) gene on protein changes of the cattle muscle tissue and its tenderness during 10-day cold storage. The analysis was performed on the longest dorsal and lumbar muscles collected from 76 bulls 6 to 12 months of age. Polymorphism identification of the above-mentioned gene was conducted using the PCR-RFLP technique. Its effect on the course of the proteolysis process was assessed by monitoring changes in proportions of tissue proteins during 10-day process of meat ageing. Special attention was focused on changes in native titin (T1) share and products of its degradation (proteins of molecular weight (m.w.) of 2400 and 200 kDa), α-actinin and protein of 37 kDa as well as myosin heavy chains (MHC). In the case of the last proteins, their polymorphism was evaluated as well. Meat tenderness was estimated measuring the value of shear force and sensorially. The highest tenderness was ascertained for the heterozygote. Its improvement was associated with a significant decrease in proportions of proteins of molecular weight of approximately 37 kDa accompanied by an increase of those with 200 kDa molecular weight. Muscles derived from cattle of CT genotype were characterised by the highest proportions of type 2a MHC isoform. Value differences between proportions determined for the heterozygote and CC and TT homozygotes of the CAPN1S gene were statistically significant. Therefore, it can be presumed that the process of meat tenderisation was especially connected with MHC polymorphism.
Similar content being viewed by others
Introduction
The proteolytic process of myofibrilar proteins plays a major role during post mortem meat tenderisation. It is associated with the action of two enzymatic systems: calpain and catepsin. The first of the systems initiates the breakup and destabilisation of the myofibrilar structure, while the second one affects partial protein degradation leading to their further proteolysis and development of tender meat. It is believed that the calpain proteolytic system plays the main role in the post mortem proteolysis and the process of meat tenderisation and a special role in this regard is assigned to μ-calpain [1, 2]. The post mortem protein proteolytic process in bovine meat is also significantly influenced by calpastatin (calpain inhibitor) which is less abundant in pork (more tender) than in beef (less tender) [3, 4].
There are many elements influencing the activity of meat proteolytic enzymes, nevertheless, special attention is paid to genetic factors [5–7]. Page et al. [8] described two mutations in the μ-calpain gene (CAPN1) which are associated with bovine meat quality. Transversion in exon 9 (C3709G) and transition in exon 14 (A4558G) were significantly correlated with bovine meat tenderness in Piemontese × Angus as well as Jersey × Limousine breeds measured 48 h post mortem. Polymorphism in intron 17 of this gene was significantly correlated with meat tenderness measured on days: 7, 14 and 21 post mortem in Brahman breed. Experiments conducted by Juszczuk-Kubiak [9] revealed a correlation of the RFLP/FokI polymorphism of the CAPN1 gene (intron 14 and exon 6—RFLP-HpyCH4IV/AgeI) with bovine meat quality, including its tenderness (exon 6—RFLP-HpyCH4IV/AgeI) and CAPN2S (3′UTR—RFLP/MboII) gene, i.e. a regulatory subunit of m-calpain with the above-mentioned traits (meat and fat content in valuable cuts, tenderness, colour, taste, consistence, pH). Recently, polymorphism of a new SNP in the 30UTR region of the bovine μ-calpain small subunit (CAPNS1) gene (exon 11—RFLP/MboII) has been discovered [10].
Therefore, it was considered appropriate to investigate its impact on the course of proteolysis and the process of bovine meat tenderisation.
Materials and methods
Analyses were performed on the thoracic and lumbar (M. longissimus thoracis and lumborum) longest muscles collected from 76 bulls of four cattle breeds (Holstein-Friesian, Polish Red, Hereford and Limousine) slaughtered at the age of 6–12 months.
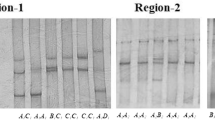
CAPN1S gene polymorphism identification of bovine muscles was performed with the assistance of the PCR-RFLP technique employing MboII endonuclease [10]. Primers with CAPN1S-F 5′-CCTCACTGTCTGTCCCTTCC-3′ and CAPN1S-R 5′-ACACAAATGTTGGGCTTGG-3′ sequences amplifying 332 bp were used in the experiment. The identification of the μ-calpain gene was performed in the 3′UTR—axon 11 region.
Samples for analyses were collected 45 min post mortem and proportions of myosin heavy chain (MHC) isoforms in fractions of rinsed myofibrils were determined employing methodology of Mozdziak et al. [11]. Changes in protein proportions of muscle tissue were evaluated 45 min post mortem and later: after 48, 72 and 240 h of cold storage using one-way electrophoresis (SDS-PAGE) in 15% polyacrylamide gel with addition of 8 M urea [12] in which the acrylamide to bis-acrylamide ratio was 199:1 [13]. From among muscle tissue proteins, particular attention was paid to the protein with the molecular weight of approximately 3700, 2400, 200, 103 and 37 kDa.
Meat tenderness measurements were conducted using an Instron type 1140 apparatus with a Warner-Bratzler attachment in accordance with methodology given by Grześ et al. [14]. Prior to value measurements of the shear force, samples were cut into slices 25–30 mm thick, vacuum-packed and heated in a water bath at the temperature of 80–81°C to reach inside temperature of 72°C and kept at the above temperature for the period of 90 min. Following their thermal treatment, samples were cooled for about half hour to room temperature and cuboids were excised measuring 10 × 10 × 40 mm which were subsequently cut perpendicularly to muscle fibres. Additionally, sensory tenderness assessment according to linear scale [15, 16] was carried out where the score of 10 points corresponded to very tender and 1 point—very tough meat. Tenderness analyses, both instrumental and sensory, were conducted on days: 2, 4 and 10 of the meat cold storage ageing.
Statistical calculations were based on the analysis of variance, whereas the significance of differences was calculated using Tukey test at the significance level of α = 0.05 using for this purpose Statistica v. 8.0 software.
Results and their discussion
Depending on genotypes, the determined proportions of native titin (T1 of 3700 kDa m.w.) in the muscle tissue of the experimental cattle ranged from 2.53 to 3.21% (Table 1) and were relatively low in comparison with data reported in other experiments [17, 18] where these proportions fluctuated from 7 to 10%. However, the above values referred to proteins of rinsed myofibrils. Since our experiments were carried out on meat proteins without their prior fractionation, the obtained values could be lower. At the same time, however, it is worth emphasising the fact that a relatively large share (from 3 to 5%) occurred in the 2400 kDa m.w. band which was very likely the product of titin degradation referred to as T2. This could indicate that the process of titin degradation in the meat of the examined bulls was relatively well advanced and its total proportion determined on the basis of the entire band comprising titin T1 and T2 ranged from 8 to 9%.
Investigations conducted by Huff-Lonergan et al. [19] showed that the majority of native titin (T1) was degraded during the period of 2 weeks. Fritz and Greaser [20] maintained that titin was still present even after 16 days of cold storage. In our studies, the recorded changes in T1 and T2 proportions during 10-day meat cold storage were small, although the proportion of T2 kept increasing gradually confirming observations made by Ho et al. [21]. The above researchers claim that this band could be noticed on meat protein separations even after 28 days of ageing both in meat after electrical stimulation and in meat which was not subjected to such treatment. In the discussed trial, the highest protein share of 2400 kDa m.w. was observed in homozygotes but differences between homozygotes and heterozygote amounted only to 0.22–0.23% and were not statistically significant (α = 0.05) (Table 1). The recorded small changes in the proportions of the two above-mentioned titin bands could have been associated with the sex of animals. It is evident from investigations conducted by Huff-Lonergan et al. [19] that the degradation process of this protein is slower in the meat of bulls which were analysed in this experiment in comparison with the meat of steers. The same researchers also suggest that the degradation process in meat of young bulls is similar to that observed in the raw material obtained from cows.
The protein proportion of 200 kDa m.w. corresponding to myosin heavy chains (MHC) and titin degradation products [22, 23] increased during the cold storage period from the initial 14% to the final level of over 16% (Table 1). This phenomenon was observed both for homozygotes and heterozygote. Statistically significant differences were determined for the heterozygote and homozygote CC. In the first case, they concerned differences between the first date of assessment (45 min) and the remaining dates, whereas in the second—only between the extreme terms of examination (Table 1). As evident from experiments conducted by Sawdy et al. [24] and Morzel et al. [25], myosin heavy chains undergo post mortem degradation the effect of which can also be the process of meat tenderisation. The observed increased proportion of the 200 kDa m.w. band at simultaneous possibility of MHC degradation makes it possible to assume that this increase could have been associated with the advancing titin degradation since products of its degradation are also observed in the band with the same molecular weight [22, 26].
The proportion of protein of 103 kDa m.w.—which corresponds to α-actinin, the main protein of Z line—during 10-day cold storage practically remained unchanged and differences observed between the initial and final values did not exceed 0.07%, irrespective of genotype (Table 1) indicating that this protein did not undergo degradation during the cold storage of the examined muscle tissue and this is in keeping with the findings of other researchers [17, 22]. The above researchers reported lack of degradation and noticeable changes in the α-actinin band during the first 14–18 days of ageing storage at the temperature of 4°C. A similar phenomenon was also observed in case of application of electrical stimulation of bovine carcasses [21]. The results of experiment conducted by Purintrapiban et al. [27] and Goll et al. [28] reveal that α-actinin is not degraded by calpains, although following interactions with other enzymes the process of its degradation is possible [27].
In the course of meat cold storage, changes were recorded in protein proportions of 37 kDa m.w. which, with respect to molecular weight, corresponds to troponin T [29–31]. This protein, from the point of view of molecular weight, may correspond to the glyceraldehyde 3-phosphate dehydrogenase—GAPDH (36–38 kDa) [32]. Beginning with the 45th minute until the 240th hour post mortem, the share of this protein decreased from 7.03 to 3.70% (Table 1). Statistically significant differences were recorded between each of the analysed dates of measurements. The process of degradation of this protein was similar for the heterozygote and homozygotes, although its course was most dynamic (decline in proportions of about 50%) for the TT homozygote and the slowest for the heterozygote. Among the analysed genotypes of the CAPN1S gene, statistically significant differences were observed in proportions of this protein between the analysed dates of ageing. In the case of the TT homozygote, these differences concerned extreme values, whereas for the heterozygote and CC homozygote, differences were recorded between three dates of cold storage. The lowest level of the 37 kDa m.w. protein following 240 h storage was observed for CC homozygote and differences between its proportions for this genotype and the heterozygote were statistically significant (Table 1). This appears to indicate similar relationships regarding changes of this protein to those observed in the case of GAPDH in pork meat [24, 32]. The above researchers maintain that its degradation may explain changes in the overall acceptability including meat taste, smell and tenderness during cold storage at 4°C. The above remark does not rule out the participation in this process of T troponin whose degradation is associated with increased meat tenderness [29–31].
When analysing the course of transformations taking place in animal muscle tissues after slaughter, which are associated with proteolysis and exert influence on its tenderness, attention is frequently focused on the character of muscle fibre metabolism [18, 33, 34] emphasising that the course of proteolysis is usually faster in muscles with the majority of type MHC 2a fibres in comparison with the red fibres (MHC 1). That is why in the course of these experiments, relationships between the changes the proteolytic process and the type of muscle fibres were analysed determining their proportions with the assistance of SDS-PAGE electrophoresis. Reports by van Hoof [35] and O’Halloran et al. [36] appear to indicate that degradation changes of muscle tissues take place faster in light fibres in comparison with the red ones, although results published by Renand et al. [37] seem to contradict these findings. One of the factors that can disturb these relationships may be the rate of the glycolytic process. Rapid rate of glycolysis, which is supported by the occurrence of type 2a and 2b MHCs, leads to the development of meat defects [38]. Type 2a MHCs are represented by fast-contracting, oxidative-glycolytic fibres. Glycolytic processes are slightly faster in them than in the type 1 red fibres rarely found in bovine muscles [39]. In the discussed experiment, the highest (38.08%) proportion of the type 2a MHCs was determined for the heterozygote of the CAPN1S gene and differences between values determined for the heterozygote and homozygotes were statistically significant (Table 2) ranging from 7.01 to 3.88%. Statistically significant differences also occurred for proportions of the type 1 MHCs between heterozygote for which their lowest level was determined (30.19%) and homozygotes (CC—40.20% and TT—39.01%, Table 2). The observed similar course of muscle protein degradation changes recorded for the compared genotypes accompanied by significant differences in MHC polymorphism may indicate that muscle fibre metabolism was a decisive factor in the meat tenderisation process.
Meat tenderness during ageing was evaluated at three terms (48, 96 and 240 h) by measuring the value of the shear force (N/cm2) and sensorially employing a linear 10-point scale. Together with the progressing process of proteolysis, starting with the 48th hour post mortem, a gradual drop in the shear force value was observed. After 48 h of ageing, the highest tenderness was determined for the CC homozygote, while already from the 4th day, the lowest shear force values were determined for heterozygote. On the last (10th) day of investigations, statistically significant differences were observed between the tenderness determined for the CC homozygote and heterozygote. Meat from the latter genotype was characterised by the highest tenderness (Table 3). When investigating the relationship of the calpain l and ll gene polymorphism with meat tenderness, Costello et al. [7] also determined its highest value for heterozygote. After 14 days of ageing, it amounted to 39.05 N/cm2 for the calpain l gene (exon 9, genotype GA) and to 43.80 N/cm2 for the gene of calpain II (regulatory unit, genotype AB).
Sensory assessment of meat tenderness reflected the results of the shear force value measurements. The highest scores corresponding to tender meat were given to the meat that was ageing for 10 days, though they were not the highest in absolute terms (maximum score was slightly above 6 points) (Table 3). These results appear to indicate that the meat of 6 to 12-month old cattle requires the same length of the ageing period to reach the desired tenderness as that of older cattle and this is in agreement with observations made by Dransfielda et al. [40] and Kołczaka et al. [17]. On the 10th day of ageing, the highest scores were awarded to heterozygote (CT) but differences between it and the remaining genotypes of the CAPN1S gene were not statistically significant (Table 3).
When analysing shear force values and scores allotted to meat samples by sensory evaluation, statistically significant differences were observed between them taking into consideration cold storage time. These differences were observed when analysing all samples, irrespective of their genotype in relation to the CAPN1S gene and when its impact was taken into consideration. However, in the first case, statistically significant differences were observed between each of the analysed dates. In the case of genotypes, they usually referred to differences between the 2nd and 10th day of meat cold storage.
Conclusions
Statistically significant impact was observed of the CAPN1S gene on meat tenderness of young slaughter cattle. The highest tenderness was determined for the heterozygote of this gene.
Meat tenderness improvement was associated with a significant decrease in the proportions of about 37 kDa m.w. proteins and increase of those with 200 kDa m.w. The recorded drop in proportions of the first group of proteins could have been associated with troponin T and GAPDH degradation, whereas the increase in the second band—with titin degradation and appearance of products of its decomposition.
Heterozygote of the CAPN1S gene was characterised by the highest share of the type 2a MHC isoform and the lowest of the type 1.
A similar course of degradation changes of muscle proteins for the compared genotypes at simultaneous significant differences in MHC polymorphism may indicate that this polymorphism associated with the impact of the CAPN1S gene was a decisive factor which determined meat tenderness.
References
Geesink GH, Kuchay S, Chishti AH, Koohmaraie M (2006) μ-Calpain is essential for postmortem proteolysis of muscle proteins. J Anim Sci 84:2834–2840
Koohmaraie M, Geesink GH (2006) Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci 74:34–43
Monin G, Ouali A (1992) Muscle differentiation and meat quality. In: Lawrie R (ed) Developments in meat science, 5th edn. Elsevier Applied Science, London, pp 89–157
Takahashi K (1996) Structural weakening of skeletal muscle tissue during post-mortem ageing of meat: the non-enzymatic mechanism of meat tenderization. Meat Sci 43:S67–S80
Koohmaraie M (1996) Biochemical factors regulating the toughening and tenderization processes of meat. Meat Sci 43(Supplement):193–201
Casas E, White SN, Wheeler TL, Schackelford SD, Koohmaraie M, Riley DG, Chale CC Jr, Jonson DD, Smith TPL (2006) Effects of calpastatin and μ-calpain markers in beef cattle and tenderness traits. J Anim Sci 84:520–525
Costello S, O’Doherty E, Troy DJ, Ernst CW, Kim K-S, Stapleton P, Sweeney T, Mullen AM (2007) Association of polymorphisms in the calpain I, calpain II and growth hormone genes with tenderness in bovine M. longissimus dorsi. Meat Sci 75:551–557
Page BT, Casas E, Heaton MP, Cullen NG, Hyndman DL, Morris CA, Crawford AM, Wheeler TL, Koohmaraie M, Keele JW, Smith TPL (2002) Evaluation of single-nucleotide polymorphisms in CAPN1 for association with meat tenderness in cattle. J Anim Sci 80:3077–3085
Juszczuk-Kubiak E (2006) Praca doktorska: Identyfikacja markerów molekularnych powiązanych z proteolizą białek w mięśniach i jakością mięsa bydła [Identification of molecular markers related to proteolysis of muscle proteins and quality of beef] IGiHZ PAN, Jastrzębiec (in Polish)
Juszczuk-Kubiak E, Flisikowski K, Wicińska K (2010) A new SNP in the 30UTR region of the bovine calpain small subunit (CAPNS1) gene. Mol Biol Rep 37:473–476
Mozdziak PE, Greaser ML, Schulz E (1998) Myogenin, MyoD and myosin expression after pharmacologically and surgically induced hypertrophy. J Appl Physiol 84:1359–1364
Pospiech E, Szalata M, van Lack RLJM, Sośnicki AA, Greaser ML (2001) Tenderness and protein changes of pork in relation on pig genotype and postmortem glycolysis phenotype. In: 47th ICoMST, Kraków, Poland, pp 258–259
Fritz JD, Swartz DR, Greaser ML (1989) Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Anal Biochem 180:205–210
Grześ B, Pospiech E, Rosochacki SJ, Łyczyński A, Mikołajczak B, Iwańska E (2007) Comparison of polymorphic myosin forms with selected meat quality attributes of bulls of various breeds and age. Pol J Food Nutr Sci 57:201–205
Adamik A (1997) Podstawowe metody analizy sensorycznej [Basic methods in sensory analysis]. Gospodarka Mięsna 11:34–37 (in Polish)
Baryłko-Pikielna N, Matuszewska I (2009) Sensoryczne badania żywności: podstawy, metody, zastosowania. [Sensory evaluation of food. Basis—Methods—Applications] Wydawnictwo Naukowe PTTŻ, Kraków, pp 163–180 (in Polish)
Kołczak T, Pospiech E, Palka K, Łącki J (2003) Changes of myofibrillar and centrifugal drip proteins and shear force of psoas major and minor and semitendinosus muscles from calves, heifers and cows during post-mortem ageing. Meat Sci 64:69–75
Huff-Lonergan E, Mitsuhashi T, Beekman DD, Parrish FC, Olson DG Jr, Robson RM (1996) Proteolysis of specific muscle structural proteins by mu-calpain at low pH and temperature is similar to degradation in postmortem bovine muscle. J Anim Sci 74:993–1008
Huff-Lonergan E, Parrish FC, Robson RM (1995) Effects of postmortem aging time, animal age, and sex on degradation of titin and nebulina in bovine longissimus muscle. J Anim Sci 73:1064–1073
Fritz JD, Greaser ML (1991) Changes in titin and nebulina in postmortem bovine musclerevealed by gel electrophoresis, western blotting and immunofluorescence microscopy. J Food Sci 56:607–610
Ho CY, Stromer MH, Robson RM (1996) Effect of electrical stimulation on postmortem titin, nebulin, desmin, and troponin-T degradation and ultrastructural changes in bovine longissimus muscle. J Anim Sci 74:1563–1575
Taylor RG, Geesing GH, Thompson VF, Koohmaraie M, Goll DE (1995) Is Z-disk degradation responsible for post-mortem tenderization? J Anim Sci 73:1351–1358
Pospiech E, Greaser M, Mikołajczak B, Szalata M, Łyczyński A (2000) Degradation and release of titin in pork muscles. In: Proceedings of the 46th ICoMST, Buenos Aires, Argentina, 2000, 4.I-P1, pp 426–427
Sawdy JC, Kaiser SA, St-Pierre NR, Wick MP (2004) Myofibrillar 1-D fingerprint and myosin heavy chain MS analyses of beef loin at 36 h postmortem correlate with tenderness at 7 days. Meat Sci 67:421–426
Morzel M, Terlouw C, Chambon CH, Micel D, Piccard B (2008) Muscle proteome and meat eating qualities of Longissimus thoracis of “Blonde d’Aquitaine” young bulls: a central role of HSP27 isoforms. Meat Sci 78:297–304
Iwanowska A, Iwańska E, Grześ B, Mikołajczak B, Pospiech E, Rosochacki SJ, Łyczyński A (2010) Changes in proteins and tenderness of meat from young bulls of four breeds at three ages over 10 days of cold storage. Anim Sci Pap Rep 28(1):13–25
Purintrapiban J, Wang M, Forsberg NE (2003) Degradation of sarcomeric and cytoskeletal proteins in cultured skeletal muscle cells. Comp Biochem Physiol B 136:393–401
Goll DE, Dayton WR, Singh I, Robson RM (1991) Studies of the a-actinin/actin interaction in the Z-disk by using calpain. J Biol Chem 266:8501–8510
Olson DG, Parrish FC, Dayton WR, Goll DE (1977) Effect of postmortem storage and calcium activated factor on the myofibrillar proteins of bovine skeletal muscle. J Food Sci 42:117–124
Penny IF, Dransfield E (1979) Relationship between toughness and troponin-T in conditioned beef. Meat Sci 3:135–141
Ho CY, Stromer MH, Robson RM (1994) Identification of the 30 kDa polypeptide in postmortem skeletal muscle as a degradation product of troponin-T. Biochimie 76:369
Okumura T, Yamada R, Nishimura T (2003) Survey of conditioning indicators for pork loins: changes in myofibrils, proteins and peptides during postmortem conditioning of vacuum-packed pork loins for 30 days. Meat Sci 64:467–473
Xiong YL (1994) Myofibrillar protein from different muscle fibre types: implication of biochemical and functional properties in meat processing. Crit Rev Food Sci Nutr 34(3):293–320
Egelandsdal B, Martinsen B (1995) Rheological parameters as predictors of protein functionality: a model study using myofibrils of different fibre-type composition. Meat Sci 39:97–111
van Hoof J (1991) Jakość mięsa w produkcji nowego typu wołowiny [Meat quality in the production of new type of beef]. Medycyna Weterynaryjna, 47(12):529–533 (in Polish)
O’Halloran GR, Troy DJ, Buckley DJ, Reville WJ (1997) The role of endogenous proteases in the tenderisation of fast glycolysing muscle. Meat Sci 47:187–210
Renand G, Picard B, Touraille C, Berge P, Lepetit L (2001) Relationships between muscle characteristics and meat quality traits of young Charolais bulls. Meat Sci 59:49–60
Grześ B, Pospiech E, Koćwin-Podsiadła M, Kurył J, Krzęcio E, Łyczyński A, Iwańska E, Mikołajczak B (2005) Effect of the RYR1 gene on meat tenderness and the polymorphism of myosin heavy chains. Ann Anim Sci Suppl 2:47–50
Picard B, Cassar-Malek I (2009) Evidence for expression of IIb myosin heavy chain isoform in some skeletal muscles of Blonde d’Aquitaine bulls. Meat Sci 82:30–36
Dransfield E, Jones RCD, MacFie HJH (1981) Tenderising in m. longissimus dorsi of beef, veal, rabbit, lamb and pork. Meat Sci 5:139–147
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Iwanowska, A., Grześ, B., Mikołajczak, B. et al. Impact of polymorphism of the regulatory subunit of the μ-calpain (CAPN1S) on the proteolysis process and meat tenderness of young cattle. Mol Biol Rep 38, 1295–1300 (2011). https://doi.org/10.1007/s11033-010-0229-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0229-5