Abstract
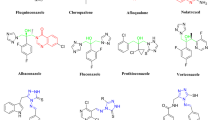
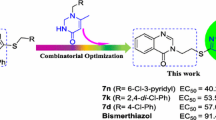
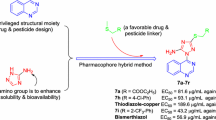
A series of novel 1,2,4-triazolo[1,5-a]pyrimidine-containing quinazolin-4(3H)-one derivatives (8a–8o) were designed, synthesized and assessed for their in vitro antibacterial and antifungal activities in agriculture. All the title compounds were completely characterized via 1H NMR, 13C NMR, HRMS and IR spectroscopic data. In particular, the molecular structure of compound 8f was further corroborated through a single-crystal X-ray diffraction measurement. The turbidimetric method revealed that some of the compounds displayed noticeable bactericidal potencies against the tested plant pathogenic bacteria. For example, compounds 8m, 8n and 8o possessed higher antibacterial efficacies in vitro against Xanthomonas oryzae pv. oryzae with EC50 values of 69.0, 53.3 and 58.9 μg/mL, respectively, as compared with commercialized agrobactericide bismerthiazol (EC50 = 91.4 μg/mL). Additionally, compound 8m displayed an EC50 value of 71.5 μg/mL toward Xanthomonas axonopodis pv. citri, comparable to control bismerthiazol (EC50 = 60.5 μg/mL). A preliminary structure–activity relationship (SAR) analysis was also conducted, based on the antibacterial results. Finally, some compounds were also found to have a certain antifungal efficacy in vitro at the concentration of 50 μg/mL.
Graphic abstract





Similar content being viewed by others
References
Sun X, Cao Y, Yang Z, Xu C, Li X, Wang S, Zhang Q (2004) Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J 37:517–527. https://doi.org/10.1046/j.1365-313X.2003.01976.x
Graham JH, Gottwald TR, Cubero J, Achor DS (2004) Xanthomonas axonopodis pv.citri: factors affecting successful eradication of citrus canker. Mol Plant Pathol 5:1–15. https://doi.org/10.1046/j.1364-3703.2003.00197.X
Huang N, Angeles ER, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennett J, Khush GS (1997) Pyramiding of bacterial blight resistance genes in rice: marker-assisted selection using RFLP and PCR. Theor Appl Genet 95:313–320. https://doi.org/10.1007/s001220050565
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G, Foster GD (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x
Wang LL, Li C, Zhang YY, Qiao CH, Ye YH (2013) Synthesis and biological evaluation of benzofuroxan derivatives as fungicides against phytopathogenic fungi. J Agric Food Chem 61:8632–8640. https://doi.org/10.1021/jf402388x
Blancard D (2012) Tomato diseases: identification, biology and control: a colour handbook, 2nd edn. Academic Press, Cambridge. https://doi.org/10.1016/C2010-0-66813-1
Yang L, Bao XP (2017) Synthesis of novel 1,2,4-triazole derivatives containing the quinazolinylpiperidinyl moiety and N-(substituted phenyl)acetamide group as efficient bactericides against the phytopathogenic bacterium Xanthomonas oryzae pv. oryzae. RSC Adv 7:34005–34011. https://doi.org/10.1039/C7RA04819J
Zhang M, Dai ZC, Qian SS, Liu JY, Xiao Y, Lu AM, Zhu HL, Wang JX, Ye YH (2014) Design, synthesis, antifungal, and antioxidant activities of (E)-6-((2-phenylhydrazono)methyl)quinoxaline derivatives. J Agric Food Chem 62:9637–9643. https://doi.org/10.1021/jf504359p
Khan I, Ibrar A, Abbas N, Saeed A (2014) Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: synthetic approaches and multifarious applications. Eur J Med Chem 76:193–244. https://doi.org/10.1016/j.ejmech.2014.02.005
Wang X, Li P, Li Z, Yin J, He M, Xue W, Chen Z, Song B (2013) Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(p-trifluoromethoxy)anilino]-4(3H)-quinazolinone moiety. J Agric Food Chem 61:9575–9582. https://doi.org/10.1021/jf403193q
Bouley R, Ding D, Peng Z, Bastian M, Lastochkin E, Song W, Suckow MA, Schroeder VA, Wolter WR, Mobashery S, Chang M (2016) Structure-activity relationship for the 4(3H)-quinazolinone antibacterials. J Med Chem 59:5011–5021. https://doi.org/10.1021/acs.jmedchem.6b00372
Qureshi SI, Chaudhari HK (2019) Design, synthesis, in-silico studies and biological screening of quinazolinone analogues as potential antibacterial agents against MRSA. Bioorg Med Chem 27:2676–2688. https://doi.org/10.1016/j.bmc.2019.05.012
Zhang J, Liu J, Ma Y, Ren D, Cheng P, Zhao J, Zhang F, Yao Y (2016) One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bioorg Med Chem Lett 26:2273–2277. https://doi.org/10.1016/j.bmcl.2016.03.052
Chen M, Li P, Hu D, Zeng S, Li T, Jin L, Xue W, Song B (2016) Synthesis, antiviral activity, 3D-QSAR, and interaction mechanisms study of novel malonate derivatives containing quinazolin-4(3H)-one moiety. Bioorg Med Chem Lett 26:168–173. https://doi.org/10.1016/j.bmcl.2015.11.006
Mohamed MA, Ayyad RR, Shawer TZ, Abdel-Aziz AAM, El-Azab AS (2016) Synthesis and antitumor evaluation of trimethoxyanilides based on 4(3H)-quinazolinone scaffolds. Eur J Med Chem 112:106–113. https://doi.org/10.1016/j.ejmech.2016.02.002
Wang H, Lee M, Peng Z, Blázquez B, Lastochkin E, Kumarasiri M, Bouley R, Chang M, Mobashery S (2016) Synthesis and evaluation of 1,2,4-triazolo[1,5-a]pyrimidines as antibacterial agents against Enterococcus faecium. J Med Chem 58:4194–4203. https://doi.org/10.1021/jm501831g
Tao X, Hu Y (2010) Synthesis and antitumor activity of 2-aryl-1, 2, 4-triazolo[1, 5-a]pyridine derivatives. Med Chem 6:65–69. https://doi.org/10.2174/157340610791321505
Bhatt JD, Chudasama CJ, Patel KD (2015) Pyrazole clubbed triazolo[1,5-a] pyrimidine hybrids as an anti-tubercular agents: Synthesis, in vitro screening and molecular docking study. Bioorg Med Chem 23:7711–7716. https://doi.org/10.1016/j.bmc.2015.11.018
Yan BR, Lv XY, Du H, Gao MN, Huang J, Bao XP (2016) Synthesis and biological activities of novel quinazolinone derivatives containing a 1,2,4-triazolylthioether moiety. Chem Pap 70:983–993. https://doi.org/10.1515/chempap-2016-0034
Yan B, Lv X, Du H, Bao X (2016) Design, synthesis and biological activities of novel quinazolinone derivative bearing 4-phenyl-5-thioxo-1,2,4-triazole Mannich bases. Chin J Org Chem 36:207–212. https://doi.org/10.6023/cjoc201506026
Du H, Fan Z, Yang L, Bao X (2018) Synthesis of novel quinazolin-4(3H)-one derivatives containing the 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety as effective agricultural bactericides against the pathogen Xanthomonas oryzae pv. oryzae. Mol Diversity 22:1–10. https://doi.org/10.1007/s11030-017-9782-3
Du H, Fan Z, Yang L, Bao X (2018) Synthesis and antimicrobial activities of novel 1,2,4-triazole-acylhydrazone derivatives containing the quinazolin-4-one moiety. Chin J Org Chem 38:531–538. https://doi.org/10.6023/cjoc201708051
Li YH, Zhang B, Yang HK, Li Q, Diao PC, You WW, Zhao PL (2017) Design, synthesis, and biological evaluation of novel alkylsulfanyl-1,2,4-triazoles as cis-restricted combretastatin A-4 analogues. Eur J Med Chem 125:1098–1106. https://doi.org/10.1016/j.ejmech.2016.10.051
Gao F, Wang T, Xiao J, Huang G (2019) Antibacterial activity study of 1,2,4-triazole derivatives. Eur J Med Chem 173:274–281. https://doi.org/10.1016/j.ejmech.2019.04.043
Yao YP, Dai FY, Dong KK, Mao Q, Wang YL, Chen T (2011) Synthesis and antibacterial activities of pleuromutilin derivatives with quinazolinone and thioether groups. J Chem Res 35:4–7. https://doi.org/10.3184/174751911X556675
Gottwald TR, Hughes G, Graham JH, Sun XA, Riley T (2001) The citrus canker epidemic in Florida: the scientific basis of regulatory eradication policy for an invasive species. Phytopathology 91:30–34. https://doi.org/10.1094/phyto.2001.91.1.30
Xu WM, Han FF, He M, Hu DY, He J, Yang S, Song BA (2012) Inhibition of tobacco bacterial wilt with sulfone derivatives containing an 1,3,4-oxadiazole moiety. J Agric Food Chem 60:1036–1041. https://doi.org/10.1021/jf203772d
Wang X, Yin J, Shi L, Zhang G, Song B (2014) Design, synthesis, and antibacterial activity of novel Schiff base derivatives of quinazolin-4(3H)-one. Eur J Med Chem 77:65–74. https://doi.org/10.1016/j.ejmech.2014.02.053
Fan ZJ, Shi J, Luo N, Ding MH, Bao XP (2019) Synthesis, crystal Structure, and agricultural antimicrobial evaluation of novel quinazoline thioether derivatives incorporating the 1,2,4-triazolo[4,3-a]pyridine moiety. J Agric Food Chem 67:11598–11606. https://doi.org/10.1021/acs.jafc.9b04733
Long QS, Liu LW, Zhao YL, Wang PY, Chen B, Li Z, Yang S (2019) Fabrication of furan-functionalized quinazoline hybrids: their antibacterial evaluation, quantitative proteomics, and induced phytopathogen morphological variation studies. J Agric Food Chem 67:11005–11017. https://doi.org/10.1021/acs.jafc.9b03419
Tao QQ, Liu LW, Wang PY, Long QS, Zhao YL, Jin LH, Xu WM, Chen Y, Li Z, Yang S (2019) Synthesis and in vitro and in vivo biological activity evaluation and quantitative proteome profiling of oxadiazoles bearing flexible heterocyclic patterns. J Agric Food Chem 67:7626–7639. https://doi.org/10.1021/acs.jafc.9b02734
Chen CJ, Song BA, Yang S, Xu GF, Bhadury PS, Jin LH, Hu DY, Li QZ, Liu F, Xue W, Lu P, Chen Z (2007) Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole 5-(3,4,5-trimethoxyphen- yl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg Med Chem 15:3981–3989. https://doi.org/10.1016/j.bmc.2007.04.014
Ye YH, Ma L, Dai ZC, Xiao Y, Zhang YY, Li DD, Wang JX, Zhu HL (2014) Synthesis and antifungal activity of nicotinamide derivatives as succinate dehydrogenase inhibitors. J Agric Food Chem 62:4063–4071. https://doi.org/10.1021/jf405437k
Dai ZC, Chen YF, Zhang M, Li SK, Yang TT, Shen L, Wang JX, Qian SS, Zhu HL, Ye YH (2015) Synthesis and antifungal activity of 1,2,3-triazole phenylhydrazone derivatives. Org Biomol Chem 13:477–486. https://doi.org/10.1039/c4ob01758g
Acknowledgements
This work was financially supported by Breeding Program of Guizhou University (No. 20185781), Young Top-Notch Talent Support Program of Guizhou Provincial Education Department (No. 2018038) and Guizhou Provincial High-Level Overseas Talents Innovation and Enterpreneurship Program (No. 201809).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, H., Ding, M., Luo, N. et al. Design, synthesis, crystal structure and in vitro antimicrobial activity of novel 1,2,4-triazolo[1,5-a]pyrimidine-containing quinazolinone derivatives. Mol Divers 25, 711–722 (2021). https://doi.org/10.1007/s11030-020-10043-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10043-z